Question 16 of 20 Submit nsider the balanced chemical reaction below. Tow many grams of excess reactant will remain when 142.0 g of CIO2 and 38.0 g of H20 react to completion? 6 CIO2(g) + 3 H;0(1) → 5 HCIO3(aq) + HCl(aq) 1 Based on your knowledge of stoichiometry, set up the table below to determine the amounts of each reactant and product after the reaction goes to completion. 6 CIO2(g) + 3 H20(1) 5 HCIO3(aq}+ HCI(aq) Before (mol) Change (mol) After (mol) 5 RESET 142.0 -142.0 38.0 -38.0 2.11 -2.11 0.351 -0.351 0.702 -0.702 1.05 -1.05 1.40 -1.40 1.75 -1.75 1.06 -1.06
Question 16 of 20 Submit nsider the balanced chemical reaction below. Tow many grams of excess reactant will remain when 142.0 g of CIO2 and 38.0 g of H20 react to completion? 6 CIO2(g) + 3 H;0(1) → 5 HCIO3(aq) + HCl(aq) 1 Based on your knowledge of stoichiometry, set up the table below to determine the amounts of each reactant and product after the reaction goes to completion. 6 CIO2(g) + 3 H20(1) 5 HCIO3(aq}+ HCI(aq) Before (mol) Change (mol) After (mol) 5 RESET 142.0 -142.0 38.0 -38.0 2.11 -2.11 0.351 -0.351 0.702 -0.702 1.05 -1.05 1.40 -1.40 1.75 -1.75 1.06 -1.06
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.69PAE: 4.69 The pictures below show a molecular-scale view of a chemical reaction between H2 and CO to...
Related questions
Question
100%
How to solve
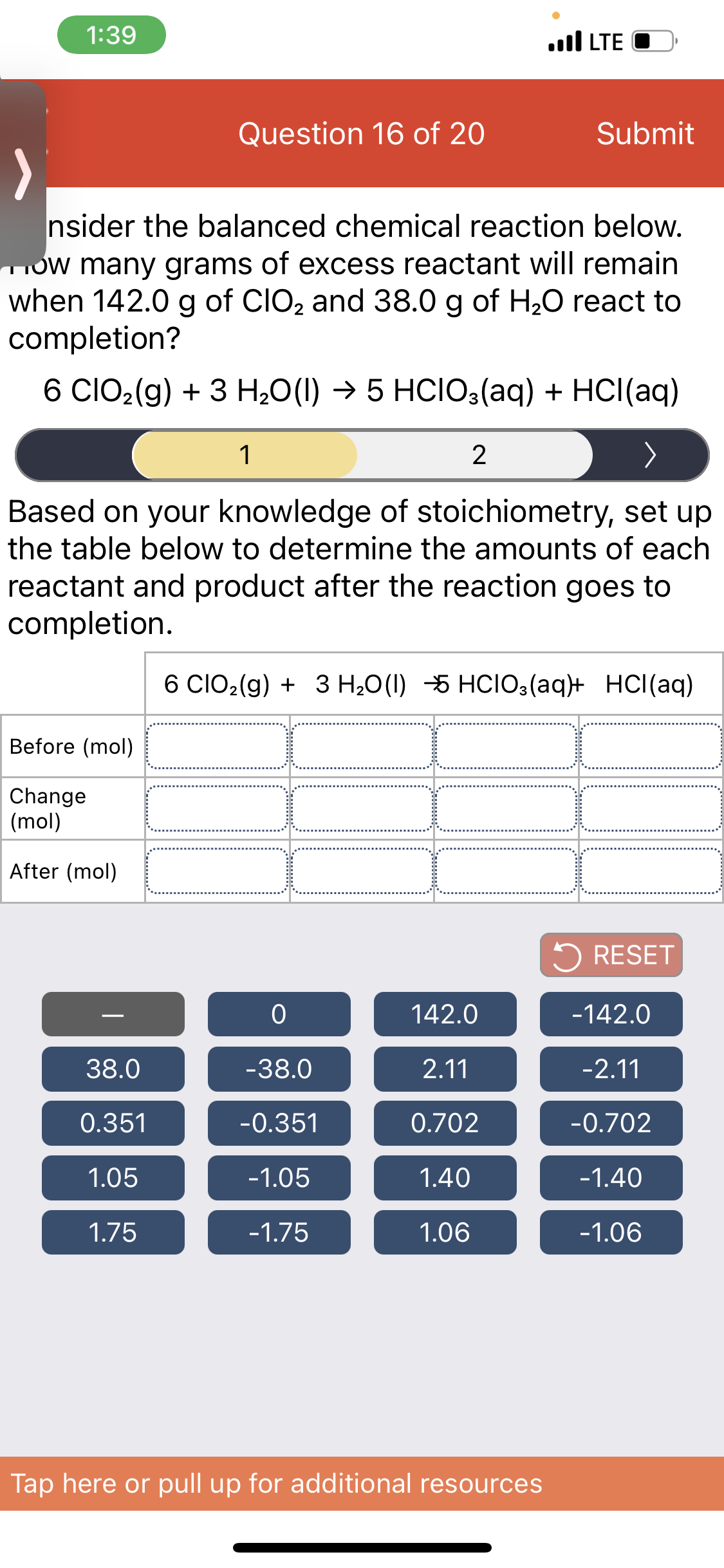
Transcribed Image Text:1:39
ull LTE
Question 16 of 20
Submit
nsider the balanced chemical reaction below.
Ow many grams of excess reactant will remain
when 142.0 g of CIO2 and 38.0 g of H2O react to
completion?
6 CIO2(g) + 3 H;0(1) → 5 HCIO3(aq) + HCl(aq)
1
Based on your knowledge of stoichiometry, set up
the table below to determine the amounts of each
reactant and product after the reaction goes to
completion.
6 CIO2(g) + 3 H,0(1) -5 HCIO3(aq+ HCl(aq)
Before (mol)
Change
(mol)
After (mol)
5 RESET
142.0
-142.0
-
38.0
-38.0
2.11
-2.11
0.351
-0.351
0.702
-0.702
1.05
-1.05
1.40
-1.40
1.75
-1.75
1.06
-1.06
Tap here or pull up for additional resources
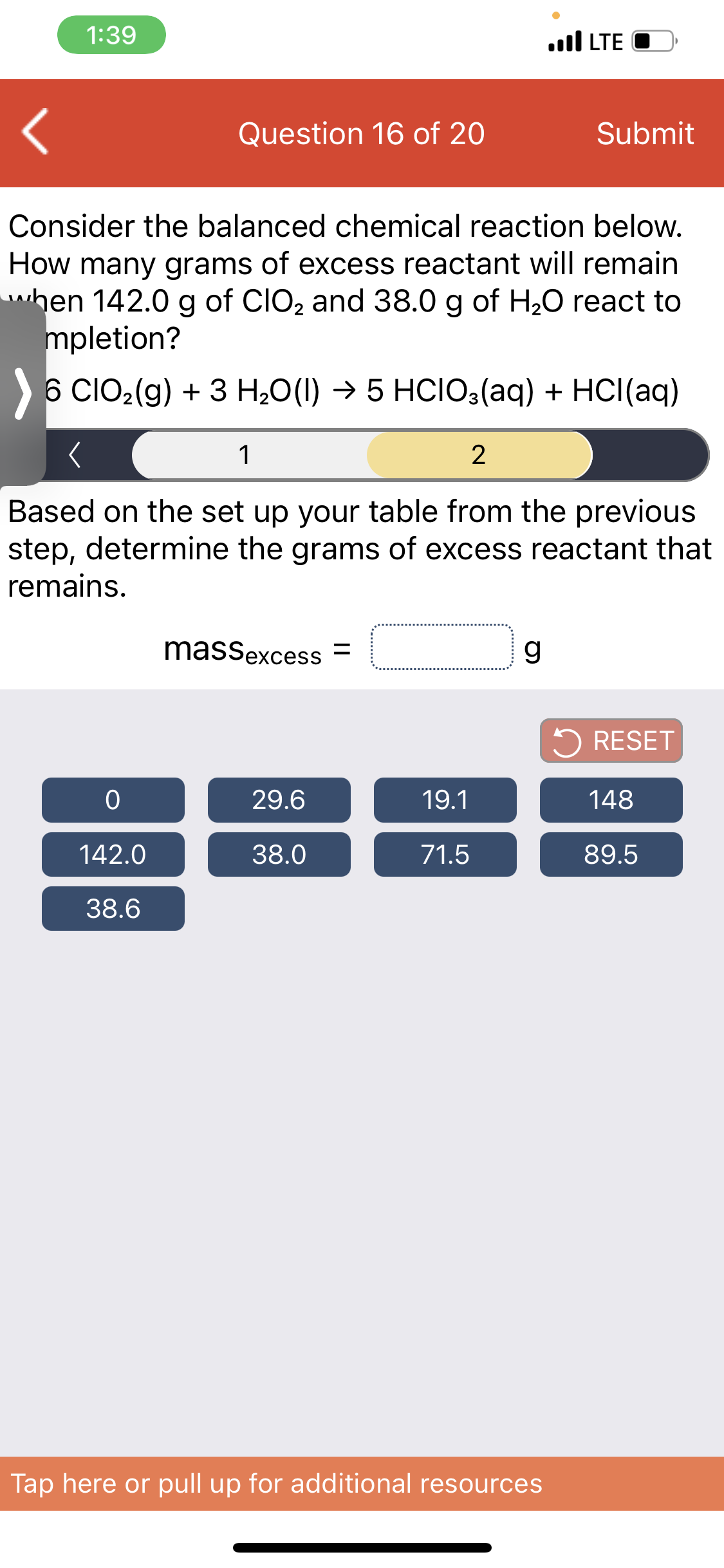
Transcribed Image Text:1:39
ull LTE
Question 16 of 20
Submit
Consider the balanced chemical reaction below.
How many grams of excess reactant will remain
hen 142.0 g of CIO2 and 38.0 g of H20 react to
mpletion?
6 CIO2(g) + 3 H20(1) → 5 HCIO3(aq) + HCl(aq)
1
Based on the set up your table from the previous
step, determine the grams of excess reactant that
remains.
massexcess =
g
5 RESET
29.6
19.1
148
142.0
38.0
71.5
89.5
38.6
Tap here or pull up for additional resources
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning