QUESTION 4 Answer the following questions based on the given table. Compound Chromophores Wavelength (Amas) Acetone C=O 186 nm and 280 nm Butene C=C 190 nm Fe(SCN)" aqueous solution of Ni²* 475 nm 400 nm a) Based on the given chromophores, determine the types of electronic transitions which occur for each of acetone and butene. b) Provide the type of electronic transition which occur for the aqueous solution of Ni". c) Iron(III) thiocyanate, Fe(SCN)* is an example of a charge-transfer complex. Outline the steps of the electron transfer process in this complex.
QUESTION 4 Answer the following questions based on the given table. Compound Chromophores Wavelength (Amas) Acetone C=O 186 nm and 280 nm Butene C=C 190 nm Fe(SCN)" aqueous solution of Ni²* 475 nm 400 nm a) Based on the given chromophores, determine the types of electronic transitions which occur for each of acetone and butene. b) Provide the type of electronic transition which occur for the aqueous solution of Ni". c) Iron(III) thiocyanate, Fe(SCN)* is an example of a charge-transfer complex. Outline the steps of the electron transfer process in this complex.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter4: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 10P: Use the data in Figure 4.8 to estimate the ratio of radiation intensity at 10,000 Å (infrared) to...
Related questions
Question
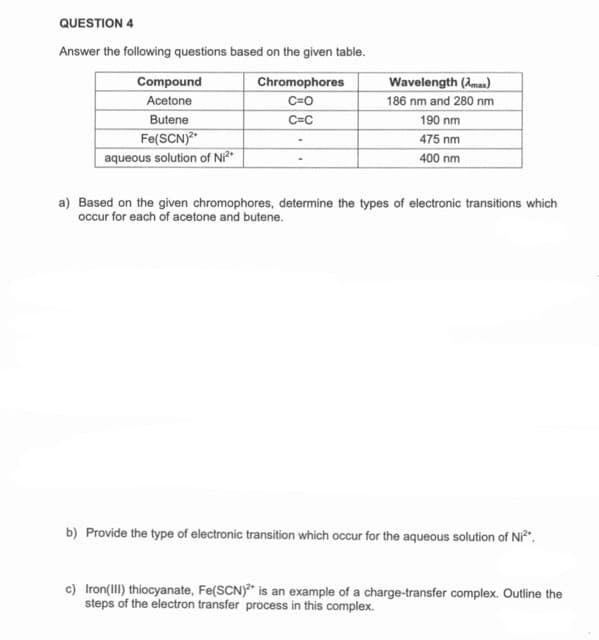
Transcribed Image Text:QUESTION 4
Answer the following questions based on the given table.
Compound
Chromophores
Wavelength (Ama)
Acetone
C=0
186 nm and 280 nm
190 nm
Butene
C=C
Fe(SCN)
aqueous solution of Ni²*
475 nm
400 nm
a) Based on the given chromophores, determine the types of electronic transitions which
occur for each of acetone and butene.
b) Provide the type of electronic transition which occur for the aqueous solution of Ni".
c) Iron(III) thiocyanate, Fe(SCN)* is an example of a charge-transfer complex. Outline the
steps of the electron transfer process in this complex.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning
