Question 6 The equilibrium constant Kc for the reaction fructose-1,6-diphosphate = glyceraldehyde-3-phosphate + dihydroxyacetone phosphate is 8.9 x 10-5 M at 25ºc and the behavior is assumed to be ideal.
Question 6 The equilibrium constant Kc for the reaction fructose-1,6-diphosphate = glyceraldehyde-3-phosphate + dihydroxyacetone phosphate is 8.9 x 10-5 M at 25ºc and the behavior is assumed to be ideal.
Chapter19: Aldehydes And Ketones: Nucleophilic Addition Reactions
Section19.13: Conjugate Nucleophilic Addition To A,b-unsaturated Aldehydes And Ketones
Problem 20P
Related questions
Question
Calculate (delta)G (naught) for the process.
Standard State: 1 M
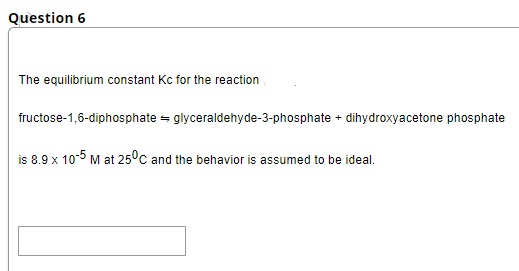
Transcribed Image Text:Question 6
The equilibrium constant Kc for the reaction
fructose-1,6-diphosphate = glyceraldehyde-3-phosphate + dihydroxyacetone phosphate
is 8.9 x 10-5 M at 25°c and the behavior is assumed to be ideal.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

