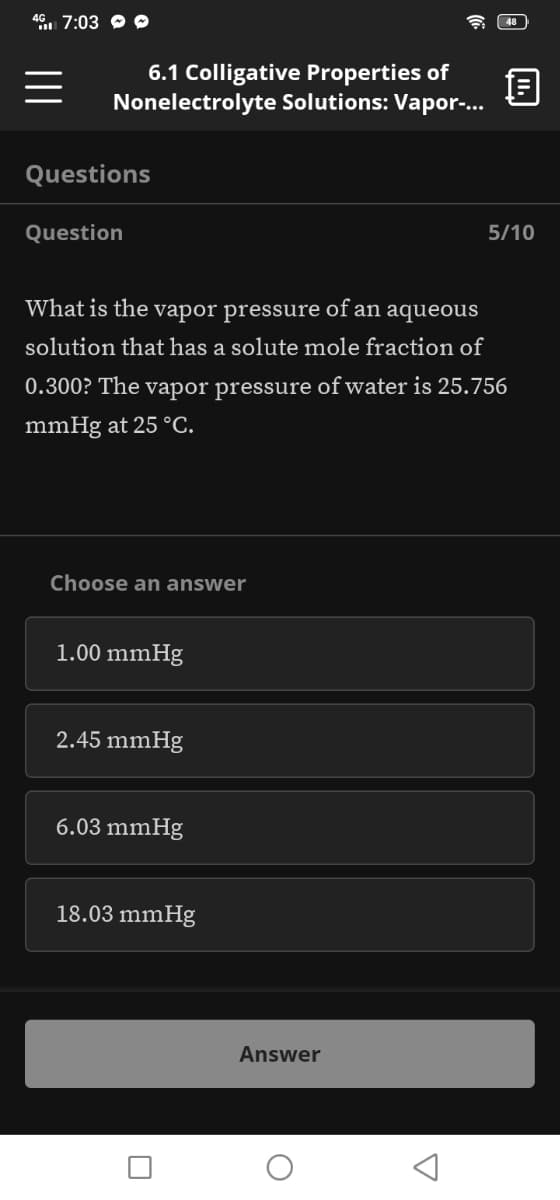
Questions Question 5/10 What is the vapor pressure of an aqueous solution that has a solute mole fraction of 0.300? The vapor pressure of water is 25.756 mmHg at 25 °C. Choose an answer 1.00 mmHg 2.45 mmHg 6.03 mmHg 18.03 mmHg
Questions Question 5/10 What is the vapor pressure of an aqueous solution that has a solute mole fraction of 0.300? The vapor pressure of water is 25.756 mmHg at 25 °C. Choose an answer 1.00 mmHg 2.45 mmHg 6.03 mmHg 18.03 mmHg
Basic Clinical Laboratory Techniques 6E
6th Edition
ISBN:9781133893943
Author:ESTRIDGE
Publisher:ESTRIDGE
Chapter6: Basic Clinical Chemistry
Section6.7: Electrolytes
Problem 1RQ
Related questions
Question
Transcribed Image Text:46. 7:03 O
48
6.1 Colligative Properties of
Nonelectrolyte Solutions: Vapor-..
Questions
Question
5/10
What is the vapor pressure of an aqueous
solution that has a solute mole fraction of
0.300? The vapor pressure of water is 25.756
mmHg at 25 °C.
Choose an answer
1.00 mmHg
2.45 mmHg
6.03 mmHg
18.03 mmHg
Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you



