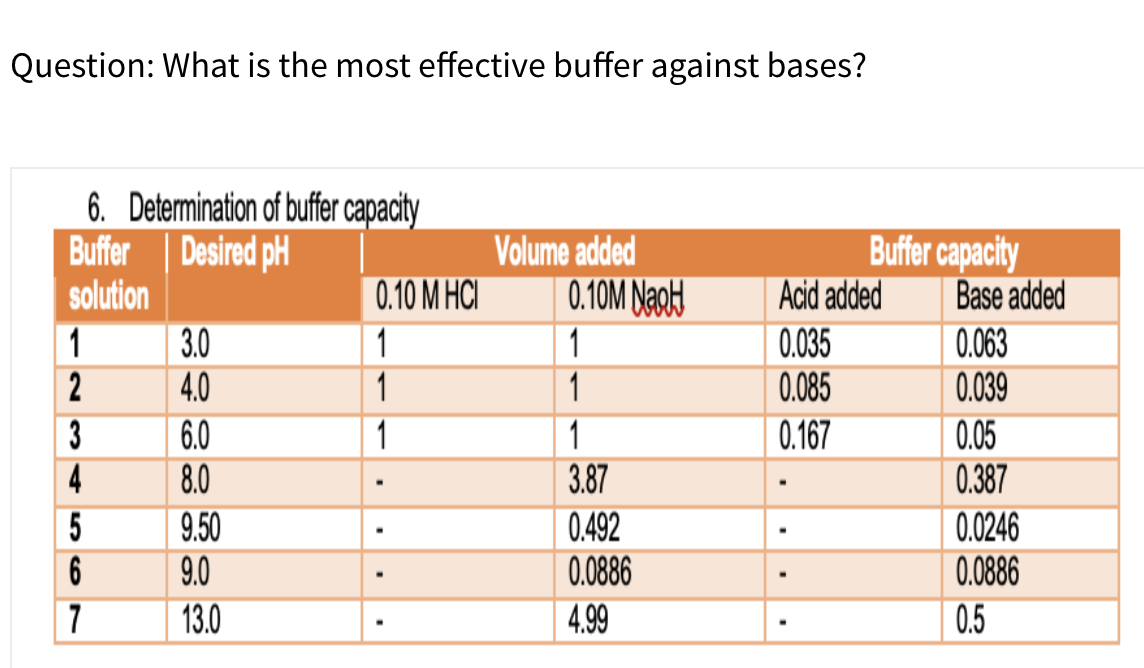
Question: What is the most effective buffer against bases? 6. Determination of buffer capacty Buffer solution Buffer capacity Acid added 0.035 0.085 0.167 Volume added 0.10M NagH Desired pH 0.10 M HCI Base added 1 2 3.0 4.0 1 1 1 1 0.063 0.039 3 4 6.0 8.0 0.05 0.387 0.0246 0.0886 1 3.87 9.50 9.0 0.492 0.0886 6 7 13.0 4.99 0.5
Q: Change in Weight Based on Concentration of Solution % Weight Change= Change F-I)/I X Solution…
A: A line graph is used to study the relationship between two different variables.
Q: 2- How much solution (in ml) of silver nitrate at a molar concentration of 0,0540 mol/l is needed as…
A: The double replacement reactions occur when ionic chemicals in a solution react with each other.…
Q: What is the pH of a solution made by mixing 25.00mL of .1500M NaOH with 25.00mL of .2500M HClO2?
A: HClO2 is Chlorous acid which is a weak acid. Weak acid dissociate weakly in aqueous solutions.…
Q: Prepare 100mL of a 2M MgO (magnesium oxide) solution. i) what is the molarity wanted? ii) what…
A: molarity is the number of moles of a molecule that is dissolved in 1000 ml or 1L here we have to…
Q: ceftazidime
A: Accurate dosing is very important in the field of healthcare. Overdosing and underdosing both are…
Q: stion 11 Calculate the amount (grams) of solid KOH to prepare 50 ml of 5 mM KOH solution (Molar…
A: Molarity It is defined as the number of moles of a substance dissolved in 1 liter of a solution.…
Q: 18. Required is 1 L of 3/8 (v/v) strength solution. The usual concentration of the solution is 12%…
A: Acetic acid solutions are made with diluting pure acetic acid with a dilutant like water. Volume by…
Q: Please answer this part: Determination of buffer capacity Buffer Buffer capacity Base added Desired…
A: Hi!Since you have posted a question with multiple subparts, we will answer the first 3 for you.…
Q: Joshua needs 150mL of 1X TBE buffer. The stock of TBE Buffer is 15X. How much 15X TBE buffer should…
A: Cells constitute all the living organisms. These cells are the building blocks of life. Cells also…
Q: 10x
A: A. 10xA difference in 1 unit in pH is equivalent to 10x H+ ion concentration.
Q: Compute for the lactic acid content of these samples given the values below. Show your solution…
A: The amount of substance present in the mixture of substances can be defined in terms of…
Q: Table 1: Absorbance data @ 500nm for Glucose standards (0-20mM) and tests solutions Tube Number…
A: Standard curve for glucose concentration is constructed by taking concentration of glucose on x axis…
Q: 2. 500 ml of a 0.1 M sodium phosphate buffer with pKa = 7.21, is diluted a) How would you dilute…
A: Normality is described as the number of gram or mole equivalents of solute present in one liter of a…
Q: 14.9 g ice cube is placed into 332 g of water. Part A Calculate the temperature change in the water…
A: First, the heat absorbed by the melting ice needs to be determined. Molar mass of water= 18.015g/mol…
Q: DATA TABLE L6108.3 nm Lamda max Trial Concentration (mol/L) Absorbance 0.428 0. 8०० 1.17 1.498 1.845…
A: Spectroscopy is the interaction between electromagnetic radiation and matter. If transmittance T…
Q: How to dilute 5x SDS stock running buffer to 300ml of 1x SDS running buffer. SDS = Sodium dodecyl…
A: Molecular biologists also make concentrated stocks of solutions to last for long periods of time in…
Q: A 5% dextrose in 1/2 normal saline (D5 1/2 NS) solution is commonly administered to patients needing…
A: IV or Intravenous fluid when injected into patients has to be carefully regulated as it would…
Q: Samples Absorbance undiluted 0.715 1:4 dilution ratio 0.447 1:9 dilution ratio 0.321 a. Calculate…
A: From the given BSA standard absorbance data, I have plotted above linear XY graph and derived Y=mx+C…
Q: Question attached
A: Molarity (M)=number of molesvolume of solution in litre=massmolecular massvolume of solution in…
Q: how to prepare a 100 mL of 4x sds buffer from a 1.0 L of 50x SDS stock solution. Show complete…
A: In order to characterize the cells genetically, it is necessary to isolate the DNA that is present…
Q: Part I. For each of the following four questions: calculate and describe how the requested solution…
A: Calculations are important to estimate the amount of solute or solution needed to make a solution of…
Q: Table of caffeine standards concentration Sample Conc, ppm Std1 16 Std2 32 Std3 48 Std4 64…
A: Given : 16 ppm std 1 concentration MM caffeine = 194.19 g/mol
Q: Based on the given results, what do you think is the isoelectric pH of casein? Briefly discuss your…
A: Casein is a key protein of milk and present in large amounts in cheese. Proteins are polymers of…
Q: Release of drug from a 20mm gel cube (B) was tested by placing it in a large dissolution apparatus…
A: In the pharmaceutical industry, the drug dissolution test is used to observe the drug release…
Q: 8. What is the molarity of the solution prepared by diluting 75 mL of 1.50 M silver nitrate (AgNO3)…
A: Molarity of a solute is the number of moles of solute per liter of solution. The concentration or…
Q: 14 strength Pulmocare 450mL q8h followed by 100mL of water after each feed. Calculate the mixture,…
A: Given, that 1/4 strength pulmocare 450 mL q8h has to be given followed by 100 mL water after every…
Q: 18 Students were provided with cubes of slightly alkaline gelatine of different dimensions,…
A: From the above data we have to explain a For each block, calculate the ratio of surface area to…
Q: Instruction: There are 4 conditions in acid-base imbalances, to understand more of this please read…
A: Oxygen is important for energy production. Oxygen is obtained from air as air enters the lungs.…
Q: Match the common laboratory scenarios to the correct buffer required using the guide below.…
A: Buffer It is defined as a solution that have the ability to resist change in pH upon the addition…
Q: 7. Bromophenol Blue, a reagent or standard that is usually used to determine the void volume of a…
A: NOTE: since you have posted multiple questions, So we will be solving the first three parts for you.…
Q: Estimate the pH of a 7.18 x 106 MHF solution. You can calculate the lowest possible pH, knowing that…
A: “Since you have asked multiple question, we will solve the first question for you. If you want any…
Q: 12 If strong-base anion exchange resin is applied to treat raw water with silicate, the pH of raw…
A: Note : Hi ! Thank you for the question. We are authorized to answer one question at a time. Since…
Q: Biol 190L Conversion & Concentration Practice Problems 1. Complete the metric system conversions…
A: Since you have not mentioned the question numbers to be solved. According to our policies, I will be…
Q: Provide a one-sentence broad generalization regarding the % acrylamide one should use in SDS PAGE…
A: Hello. Since your question has multiple parts, we will solve the first question for you. If you want…
Q: The package says aspirin 325 mg. I need to figure out how many tablets I need to administer In…
A: Here, The package says aspirin 325 mg, 1 grain is equivalent to approximately 65 mg Now, Set up…
Q: questions. Generic Standard Curve 0.7 y = 0.9425x- 0.0034 0.6 0.5 04 0.3 02 0.1 0.1 02 0.3 0.4 0.5…
A: The standard curve represents the relationship between absorbance and concentration. The standard…
Q: What is the chemistry principle behind when (treatment: heavy cream) is subjected to CHILLED…
A: Here the experiment is basically the chilling effect on heavy cream and studing the pattern of its…
Q: A 1.00 g sample containing NaHCO3 was dissolved in water and titrated using 0.500 M HCl. The sample…
A: A chemical laboratory method is used for the quantitative analysis of an analyte that is already…
Q: New December 18, 2020. The nitrite in a series of standard solutions (mg/L, n = 5) are converted to…
A: Absorbance is the fraction of intensity of light that is absorbed by a material . According to…
Q: C. Results Match each beaker with the set-up description in the Table 1 below based on the expected…
A: Acid is the substance that donates its proton, and an example of the acid is hydrochloric acid. The…
Q: Give the complete solution.
A: Using the Henderson-Hasselbach Equation pH=pKa +log[base]/[acid]------(1) or pH=pka + log[A-]/[HA]…
Q: concentration? 5% dextrose in a 0.45% 5% dextrose in water 0.9% normal saline 0.45% normal saline. A…
A: The IV solution is the fluid of choice for patients with several trauma or deficiencies. It is used…
Q: Concentration Buffer solution pka Desired ph 0.50 M Acetate 4.70 5.0 Using assigned buffer determine…
A: Buffers are solutions with the ability to prevent a change in pH when acids or basses are added to…
Q: It required 66.66 mL of 2.000 M HBrO3 to titrate (neutralize) 50.00 mL of an AI(OH)3 solution. What…
A: In a chemical reaction, two or more reactants react with each other to form new substances. This new…
Q: 2. Calculate the following: a. you are asked to prepare 10 NA slants and 5 NA stab in big tubes. How…
A: Growth media also known as culture media are used to cultivate microorganisms. These media are a…
Q: 25. What would be the new pH of a 1L buffer solution (pH=7.8) upon the addition of 3mL O.0001 M HCI.…
A: A buffer solution is an aqueous mixture of a weak acid and its conjugate base, or vice versa, known…
Q: Blood is an alkaline buffer having pH around 7.4. If blood would not be a buffer, we may not…
A: Homeostasis is a tendency to maintain the stability of physical and chemical parameters that enable…
Q: A mixture containing only KCl and NaBr is analyzed by the Mohr Method. A 0.3172-g sample is…
A: NaBr is generally cerated by treating sodium hydroxide with hydrogen bromide. Sodium bromide is…
Q: Compare the solubility of lead carbonate in each of the following aqueous solutions: Clear All 0.10…
A: Solubility of lead carbonate in Pb(NO3)2 and K2CO3 will be less than it's solubility in pure water.…
pls answer
Step by step
Solved in 2 steps

- A mixture containing only KCl and NaBr is analyzed by the Mohr Method. A 0.3172-g sample is dissolved in 50 mL water and titrated to the Ag2CrO4 endpoint, requiring 36.85 mL of 0.1120 M AgNO3. A blank titration requires 0.71 mL of titrant to reach the same endpoint. Report the % (w/w) KCl and NaBr in the sample. [Ans . 84.41 % (w /w); 17.59 % (w /w)]The dilution factor for Sample 1 if 20 µL of extract is added to 980 µL distilled water is 50. What would the dilution factor for Sample 1 be if 500 µL of the diluted sample from last sentence were diluted with 500 µL distilled water?Prepare 100mL of a 2M MgO (magnesium oxide) solution. i) what is the molarity wanted? ii) what is the total volume of solution wanted (in LITERS)? iii) What is the molar mass of MgO? iv) What is the mass of MgO (solute) needed? v) What is the volume of water (solvent) needed?
- What is the chemistry principle behind when (treatment: heavy cream) is subjected to room temperature? Why does beating time, stability (%drain) and volume foam (specific gravity) is important? see the data below Treatment (Heavy Cream) Beating Time (min) Stability (%drain) Volume of Foam (Specific Gravity) (Room Temperature) 20 No drain 0.94 Heavy Cream (chilled) 20 No drain 0.76 Heavy Cream with sugar and vanilla 20 No drain 0.88 Heavy Cream (over-whipped) 25 No drain 0.83Calculate the pH of a buffer that contains 0.15 M MOPS anionic form and 0.25M MOPS zwitterion. The pKa is 7.2A prescription asked for a 500 mL 12% dextrose solution. How would you prepare the prescription if the available dextrose solutions in the pharmacy are 10% and 20%?
- I need the full answer... At 10 am you received a doctors order that you have to Infuse 1,000ml of PNSS in 10 hours to Mr Dela Cruz. What is the correct rate of this isotonic solution when the drop factor is 10 gtt = 1ml ? 15.73 qtts/min 31.249 qtts/min 49 qtts/min 18 qtts/minThe doctor ordered Oxacillin 650mg every 6 hours. The instructions on the 2gm vial states to reconstitute with powder with 11.5ml of sterile water for injection, yielding 220mg/1.5ml. How many ml will you administer? using dimensional analysis and show workTable of caffeine standards concentration Sample Conc, ppm Std1 16 Std2 32 Std3 48 Std4 64 Std5 80 If the volume used to make 100 mL of std 1 is 2 uL what is the concentration in M used to make a standard calibration curve? The standards are going to be used to build calibration curve to analyze caffeine in an energy drink. If 500 mL of the energy drink has target of 400 mg caffeine, how will you prepare the sample if you need 10 mL for the analysis ? . Caffeine MM=194.19 g/mol.
- My data is attached, please 1.) Create a scatter chart with all the data then **Using your graph - predict how many seconds it will take to dissolve an Alka-Seltzer® tablet in 200 mL of water at the following temperatures. Use the exponential trend line to find the function used in predicting the time. If needed, please refer to the Introduction to Graphing manual for guidance. 15°C, 35°C and 60°CWhat is the chemistry principle behind when (treatment: heavy cream) is subjected to CHILLED TEMPERATURE? What is the effect in beating time, stability (%drain) and volume foam (specific gravity) is important? see the data below Treatment (Heavy Cream) Beating Time (min) Stability (%drain) Volume of Foam (Specific Gravity) (Room Temperature) 20 No drain 0.94 Heavy Cream (chilled) 20 No drain 0.76 Heavy Cream with sugar and vanilla 20 No drain 0.88 Heavy Cream (over-whipped) 25 No drain 0.832 if there is a stock solution of 2000 (ug/ml) , is it possible to use SERIAL DILUTION to generate the following 8 tubes with their concentrations? A 2000 (ug/ml) B 1500 (ug/ml) C 1000 (ug/ml) D 750 (ug/ml) E 500 (ug/ml) F 250 (ug/ml) G 125 (ug/ml) H 0 (ug/ml)





