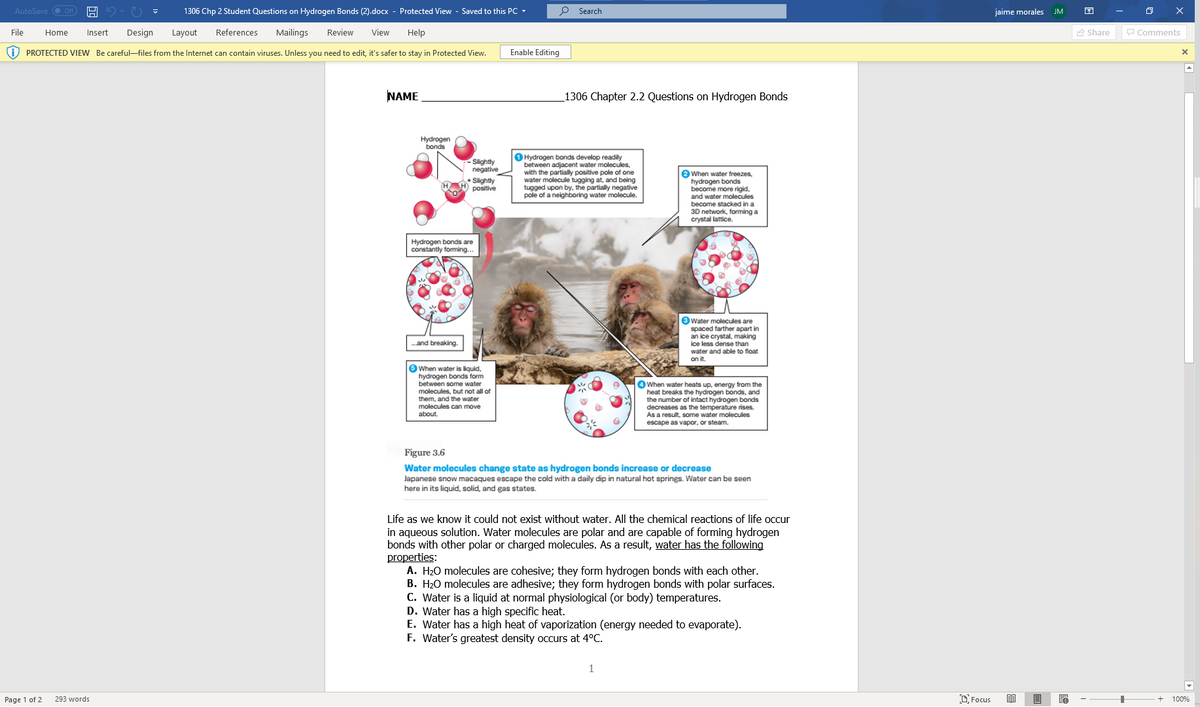
Questions: Explain how these properties of water are related to the phenomena described in parts A-H below. More than one property may be used to explain a given phenomenon. 1. During the winter, air temperature in the northern United States can remain below 0°C for months; however, the fish and other animals living in the lakes survive. 2. Many substances – for example, salt (NaCI) and sucrose - dissolve quickly in water. 3. When you pour water into a 25-ml graduated cylinder, a meniscus forms at the top of the water column. 4. Sweating and the evaporation of sweat from the body surface help reduce a human's body temperature. 5. Water drops that fall on your car tend to bead up or round up more after you polish (or wax) that car than before you polished it. 6. If you touch the edge of a paper towel to a drop of colored water, the water will move up into (or be absorbed by) the towel. Answers: Type the correct letter(s) next to the appropriate number. 1. 2. 3.
Nucleotides
It is an organic molecule made up of three basic components- a nitrogenous base, phosphate,and pentose sugar. The nucleotides are important for metabolic reactions andthe formation of DNA (deoxyribonucleic acid) and RNA (ribonucleic acid).
Nucleic Acids
Nucleic acids are essential biomolecules present in prokaryotic and eukaryotic cells and viruses. They carry the genetic information for the synthesis of proteins and cellular replication. The nucleic acids are of two types: deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). The structure of all proteins and ultimately every biomolecule and cellular component is a product of information encoded in the sequence of nucleic acids. Parts of a DNA molecule containing the information needed to synthesize a protein or an RNA are genes. Nucleic acids can store and transmit genetic information from one generation to the next, fundamental to any life form.
Hi can you answer the questions 1 through 6. On the first page is help to answer the questions on the other page.

Trending now
This is a popular solution!
Step by step
Solved in 2 steps









