R ter shift We will begin the anion analysis with a known solution containing I-, Cl-, and SO42-. First, we add aqueous silver nitrate to the known anion solution and the separation begins. Figure 3 presents an overview of the analysis. In Step 1, I- is confirmed; in Step 2, Cl- is confirmed; and in Step 3, SO42- is confirmed. STEP 1 Ag.NO3 7.cr,so2- STEP 3 Ba(NO3)2 Decant white BaSO4 ppt yellow Agl. AgC1 ppt (CONFIRMS SO2) NH4OH yellow Agl. AgCl ppt STEP 2 litmus HNO, paper (T Decant white Agl, Agl ppt yellow Agl ppt (CONFIRMS CF) (CONFIRMSI") red spot Figure 3 Anion Analysis The systematic separation and identification of I-, CF, and SO42- anions in a known solution. 16 10 14 num backspace lock & %24 7. { home enter 5. Refer to Figure 3, and determine which of the following anions are present and absent in an unknown anion solution: I, CF, and SO42-. • Unknown solution in test tube #1 plus aqueous AGNO3 gives a yellow precipitate. • The supernate in test tube #1 is poured into test tube #3. • The yellow precipitate in test tube #1 does not dissolve in aqueous NH4OH. • The supernate in test tube #1 is decanted into test tube #2. • Test tube #2 plus aqueous HNO3 gives no reaction. • Test tube #3 plus aqueous Ba(NO3)2 yields a white precipitate. Anion(s) present Anion(s) absent What safety precautions should be taken while performing this experiment? 00 く
R ter shift We will begin the anion analysis with a known solution containing I-, Cl-, and SO42-. First, we add aqueous silver nitrate to the known anion solution and the separation begins. Figure 3 presents an overview of the analysis. In Step 1, I- is confirmed; in Step 2, Cl- is confirmed; and in Step 3, SO42- is confirmed. STEP 1 Ag.NO3 7.cr,so2- STEP 3 Ba(NO3)2 Decant white BaSO4 ppt yellow Agl. AgC1 ppt (CONFIRMS SO2) NH4OH yellow Agl. AgCl ppt STEP 2 litmus HNO, paper (T Decant white Agl, Agl ppt yellow Agl ppt (CONFIRMS CF) (CONFIRMSI") red spot Figure 3 Anion Analysis The systematic separation and identification of I-, CF, and SO42- anions in a known solution. 16 10 14 num backspace lock & %24 7. { home enter 5. Refer to Figure 3, and determine which of the following anions are present and absent in an unknown anion solution: I, CF, and SO42-. • Unknown solution in test tube #1 plus aqueous AGNO3 gives a yellow precipitate. • The supernate in test tube #1 is poured into test tube #3. • The yellow precipitate in test tube #1 does not dissolve in aqueous NH4OH. • The supernate in test tube #1 is decanted into test tube #2. • Test tube #2 plus aqueous HNO3 gives no reaction. • Test tube #3 plus aqueous Ba(NO3)2 yields a white precipitate. Anion(s) present Anion(s) absent What safety precautions should be taken while performing this experiment? 00 く
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.1QAP
Related questions
Question
Transcribed Image Text:R
ter
shift
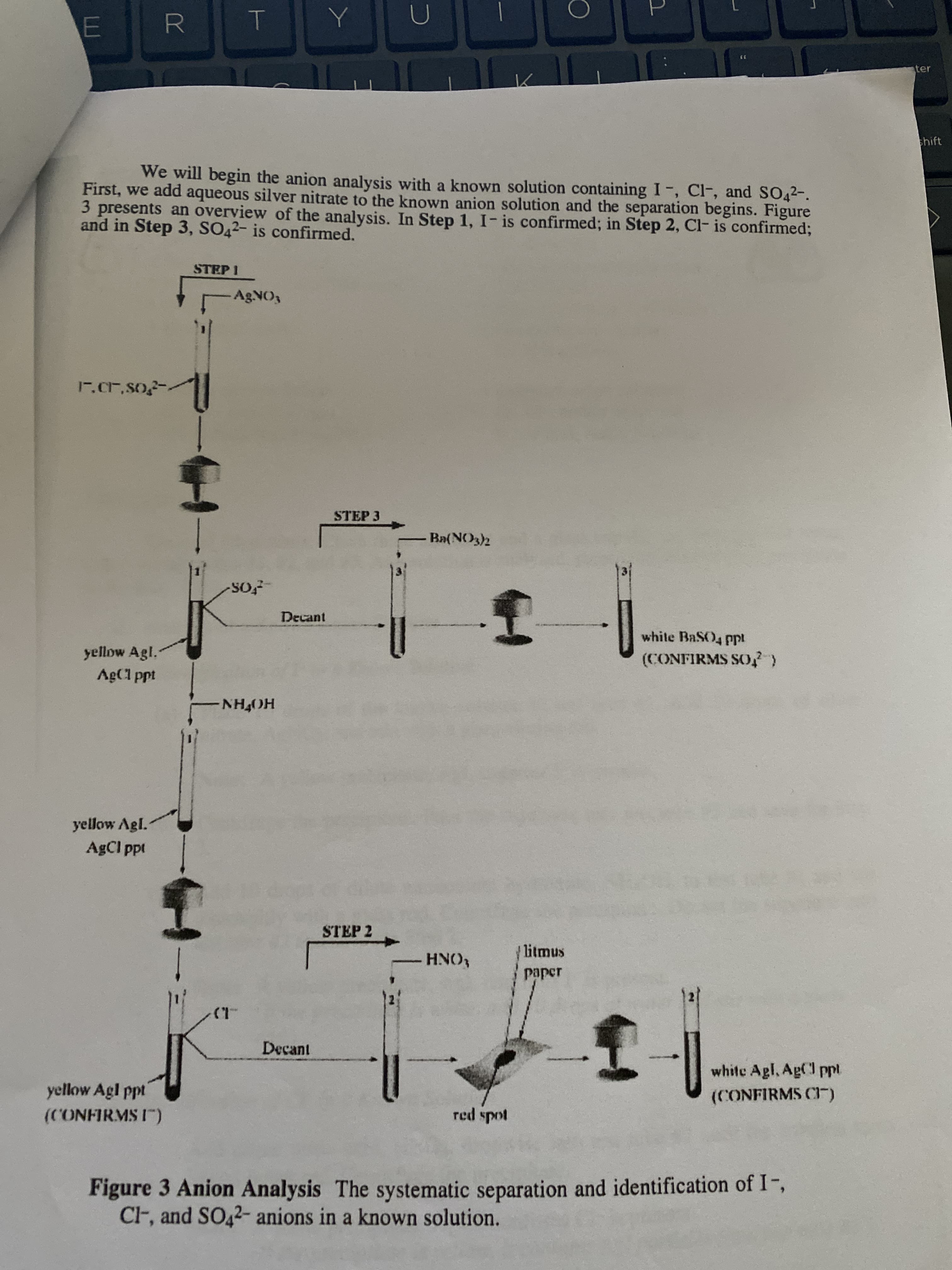
We will begin the anion analysis with a known solution containing I-, Cl-, and SO42-.
First, we add aqueous silver nitrate to the known anion solution and the separation begins. Figure
3 presents an overview of the analysis. In Step 1, I- is confirmed; in Step 2, Cl- is confirmed;
and in Step 3, SO42- is confirmed.
STEP 1
Ag.NO3
7.cr,so2-
STEP 3
Ba(NO3)2
Decant
white BaSO4 ppt
yellow Agl.
AgC1 ppt
(CONFIRMS SO2)
NH4OH
yellow Agl.
AgCl ppt
STEP 2
litmus
HNO,
paper
(T
Decant
white Agl, Agl ppt
yellow Agl ppt
(CONFIRMS CF)
(CONFIRMSI")
red spot
Figure 3 Anion Analysis The systematic separation and identification of I-,
CF, and SO42- anions in a known solution.

Transcribed Image Text:16
10
14
num
backspace
lock
&
%24
7.
{
home
enter
5. Refer to Figure 3, and determine which of the following anions are present and absent in
an unknown anion solution: I, CF, and SO42-.
• Unknown solution in test tube #1 plus aqueous AGNO3 gives a yellow precipitate.
• The supernate in test tube #1 is poured into test tube #3.
• The yellow precipitate in test tube #1 does not dissolve in aqueous NH4OH.
• The supernate in test tube #1 is decanted into test tube #2.
• Test tube #2 plus aqueous HNO3 gives no reaction.
• Test tube #3 plus aqueous Ba(NO3)2 yields a white precipitate.
Anion(s) present
Anion(s) absent
What safety precautions should be taken while performing this experiment?
00
く
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

