1 pause ↑ shift pg dn end del Mg2+. Ammonium sulfate is added to the known cation solution and the separation begins. Figure 4 presents an overview of the analysis. In Step 1, Ba2+ is confirmed; in Step 2, Ca2+ is confirmed; and in Step 3, Mg2+ is confirmed. We will begin the cation analysis with a known solution containing Ba2+, Ca2+, and itel) STEP 1 (NH4)2SO4 RE 15 TH GEN Ba2, Ca2, Mg2+ STEP 2 (NH4)2C2O4 Ca2+, Mg2+ Decant BaSO4 ppt HCI STEP 3 NA2HPO4 + NAOH Green Flame Test Mg2+ 3 (CONFIRMS Ba2+) Decant CaC204 ppt НС Brick-Red Flame Test (CONFIRMS Ca2+) 31 Discard supernate HCl and magnesium indicator 3 red litmus NAOH 3/ Blue "Lake" ppt (CONFIRMS Mg²+) blue spot Figure 4 Cation Analysis The systematic separation and identification of Ba2+, Ca2+, and Mg2+ cations in a known solution. Identifying Cations in Solution 78 3. Σ home 4. enter 3. 1 pause 1 shift pg dn end enter del ins alt Refer to Figure 4, and determine which of the following cations are present and absent in Reknown cation solution: Ba2+, Ca2+, and Mg2+ Unknown solution in test tube #1 plus aqueous (NH4)2SO4 gives a white precipitate. The supernate in test tube #1 is poured into test tube #2. • The precipitate in test tube #1 plus HCl gives a green flame test for two seconds. • The solution in test tube #2 plus aqueous (NH4)2C204 gives no reaction. • The solution in test tube #2 is poured into test tube #3. • Test tube #3 plus aqueous N22HPO4 and NaOH gives a white precipitate. • The precipitate dissolves in HCI, and magnesium indicator is added. • The solution is made basic with aqueous NAOH and centrifuged. • A blue "lake," that is a clear blue gel, is observed at the bottom of the test tube. Cation(s) absent Cation(s) present rforming this experiment? 2N
1 pause ↑ shift pg dn end del Mg2+. Ammonium sulfate is added to the known cation solution and the separation begins. Figure 4 presents an overview of the analysis. In Step 1, Ba2+ is confirmed; in Step 2, Ca2+ is confirmed; and in Step 3, Mg2+ is confirmed. We will begin the cation analysis with a known solution containing Ba2+, Ca2+, and itel) STEP 1 (NH4)2SO4 RE 15 TH GEN Ba2, Ca2, Mg2+ STEP 2 (NH4)2C2O4 Ca2+, Mg2+ Decant BaSO4 ppt HCI STEP 3 NA2HPO4 + NAOH Green Flame Test Mg2+ 3 (CONFIRMS Ba2+) Decant CaC204 ppt НС Brick-Red Flame Test (CONFIRMS Ca2+) 31 Discard supernate HCl and magnesium indicator 3 red litmus NAOH 3/ Blue "Lake" ppt (CONFIRMS Mg²+) blue spot Figure 4 Cation Analysis The systematic separation and identification of Ba2+, Ca2+, and Mg2+ cations in a known solution. Identifying Cations in Solution 78 3. Σ home 4. enter 3. 1 pause 1 shift pg dn end enter del ins alt Refer to Figure 4, and determine which of the following cations are present and absent in Reknown cation solution: Ba2+, Ca2+, and Mg2+ Unknown solution in test tube #1 plus aqueous (NH4)2SO4 gives a white precipitate. The supernate in test tube #1 is poured into test tube #2. • The precipitate in test tube #1 plus HCl gives a green flame test for two seconds. • The solution in test tube #2 plus aqueous (NH4)2C204 gives no reaction. • The solution in test tube #2 is poured into test tube #3. • Test tube #3 plus aqueous N22HPO4 and NaOH gives a white precipitate. • The precipitate dissolves in HCI, and magnesium indicator is added. • The solution is made basic with aqueous NAOH and centrifuged. • A blue "lake," that is a clear blue gel, is observed at the bottom of the test tube. Cation(s) absent Cation(s) present rforming this experiment? 2N
Chapter16: Solubility And Complex Ion Equilibria
Section: Chapter Questions
Problem 7RQ
Related questions
Question
Transcribed Image Text:1
pause
↑ shift
pg dn
end
del
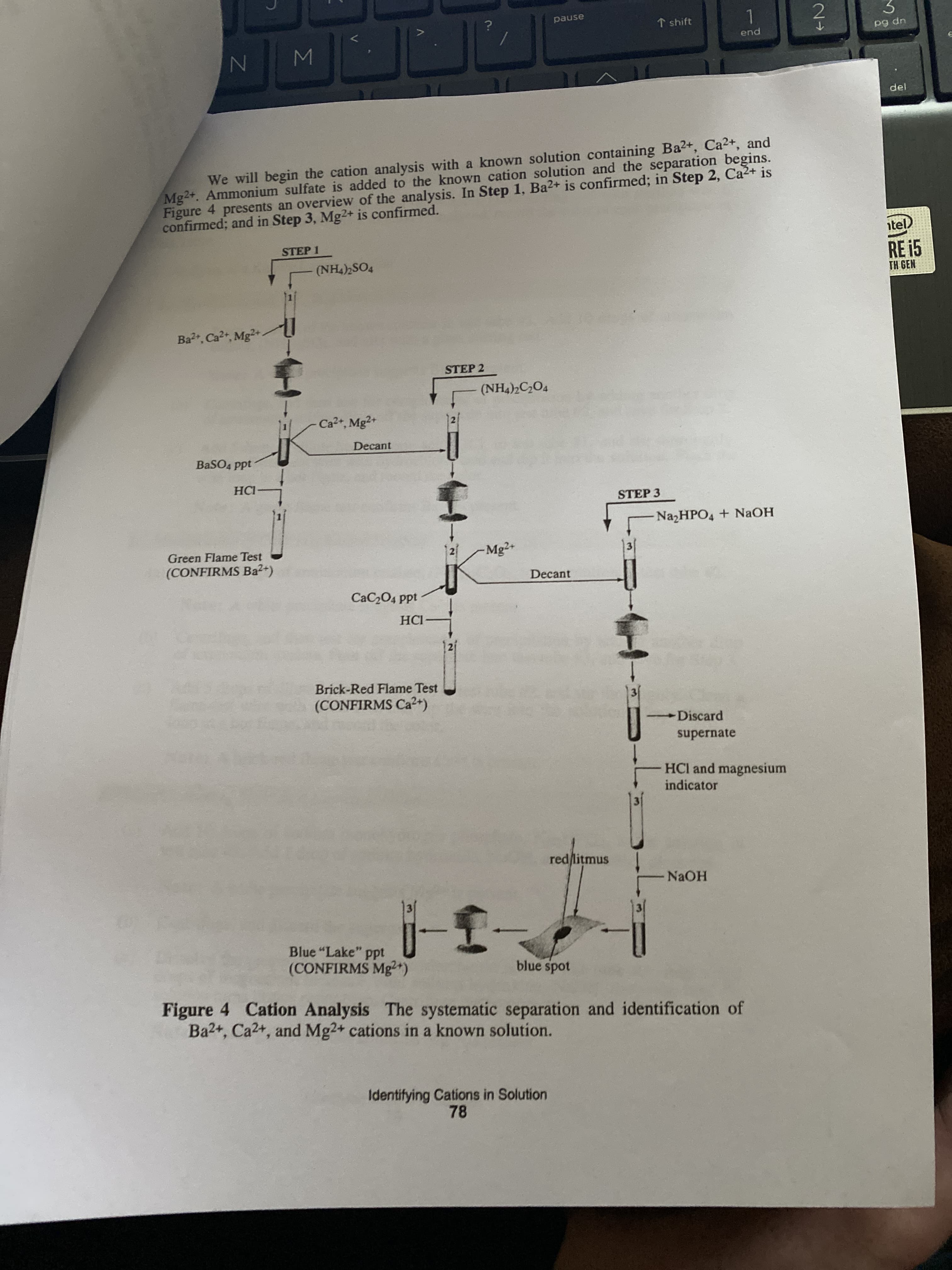
Mg2+. Ammonium sulfate is added to the known cation solution and the separation begins.
Figure 4 presents an overview of the analysis. In Step 1, Ba2+ is confirmed; in Step 2, Ca2+ is
confirmed; and in Step 3, Mg2+ is confirmed.
We will begin the cation analysis with a known solution containing Ba2+, Ca2+, and
itel)
STEP 1
(NH4)2SO4
RE 15
TH GEN
Ba2, Ca2, Mg2+
STEP 2
(NH4)2C2O4
Ca2+, Mg2+
Decant
BaSO4 ppt
HCI
STEP 3
NA2HPO4 + NAOH
Green Flame Test
Mg2+
3
(CONFIRMS Ba2+)
Decant
CaC204 ppt
НС
Brick-Red Flame Test
(CONFIRMS Ca2+)
31
Discard
supernate
HCl and magnesium
indicator
3
red litmus
NAOH
3/
Blue "Lake" ppt
(CONFIRMS Mg²+)
blue spot
Figure 4 Cation Analysis The systematic separation and identification of
Ba2+, Ca2+, and Mg2+ cations in a known solution.
Identifying Cations in Solution
78
3.
Σ

Transcribed Image Text:home
4.
enter
3.
1
pause
1 shift
pg dn
end
enter
del
ins
alt
Refer to Figure 4, and determine which of the following cations are present and absent in
Reknown cation solution: Ba2+, Ca2+, and Mg2+
Unknown solution in test tube #1 plus aqueous (NH4)2SO4 gives a white precipitate.
The supernate in test tube #1 is poured into test tube #2.
• The precipitate in test tube #1 plus HCl gives a green flame test for two seconds.
• The solution in test tube #2 plus aqueous (NH4)2C204 gives no reaction.
• The solution in test tube #2 is poured into test tube #3.
• Test tube #3 plus aqueous N22HPO4 and NaOH gives a white precipitate.
• The precipitate dissolves in HCI, and magnesium indicator is added.
• The solution is made basic with aqueous NAOH and centrifuged.
• A blue "lake," that is a clear blue gel, is observed at the bottom of the test tube.
Cation(s) absent
Cation(s) present
rforming this experiment?
2N
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
