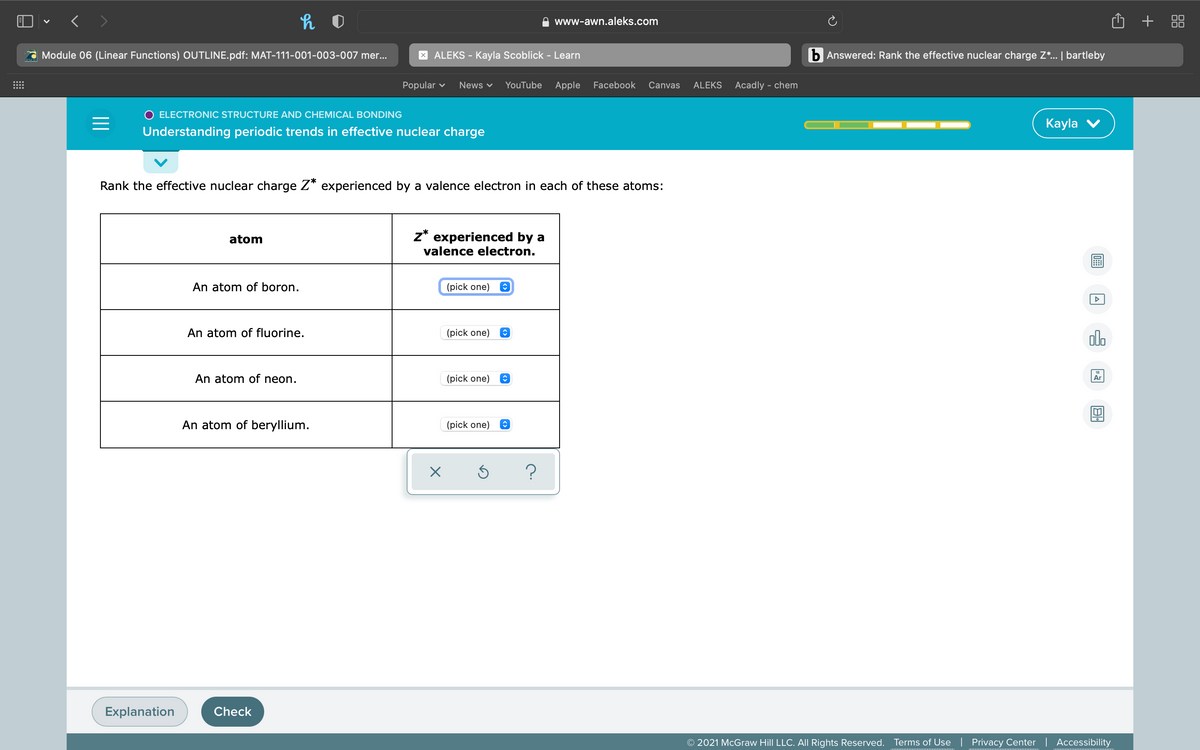
Rank the effective nuclear charge Z* experienced by a valence electron in each of these atoms: z* experienced by a valence electron. atom An atom of boron. (pick one) e An atom of fluorine. (pick one) e An atom of neon. (pick one) e An atom of beryllium. (pick one) e ?
Rank the effective nuclear charge Z* experienced by a valence electron in each of these atoms: z* experienced by a valence electron. atom An atom of boron. (pick one) e An atom of fluorine. (pick one) e An atom of neon. (pick one) e An atom of beryllium. (pick one) e ?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter6: Quantum Mechanics And Molecular Structure
Section: Chapter Questions
Problem 68AP: The Be2 molecule has been detected experimentally. It has a bond length of 2.45 Å and a bond...
Related questions
Question
Transcribed Image Text:O + 88
www-awn.aleks.com
Module 06 (Linear Functions) OUTLINE.pdf: MAT-111-001-003-007 mer...
X ALEKS - Kayla Scoblick - Learn
b Answered: Rank the effective nuclear charge Z*... | bartleby
Popular v
News v
YouTube
Apple
Facebook
Canvas
ALEKS
Acadly - chem
O ELECTRONIC STRUCTURE AND CHEMICAL BONDING
Кayla
Understanding periodic trends in effective nuclear charge
Rank the effective nuclear charge Z* experienced by a valence electron in each of these atoms:
atom
z* experienced by a
valence electron.
An atom of boron.
(pick one)
An atom of fluorine.
(pick one)
alo
An atom of neon.
(pick one)
Ar
An atom of beryllium.
(pick one)
Explanation
Check
© 2021 McGraw Hill LLC. All Rights Reserved.
Terms of Use | Privacy Center | Accessibility
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,