Rank the following compounds in order of decreasing acidity, putting the most acidic first. CH4 NH3 HF H20 I II III IV Multiple Choice IV > || > III > I III > || > IV > I |> || > IV > II III > IV > || > |
Rank the following compounds in order of decreasing acidity, putting the most acidic first. CH4 NH3 HF H20 I II III IV Multiple Choice IV > || > III > I III > || > IV > I |> || > IV > II III > IV > || > |
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter4: Polar Bonds, Polar Reactions
Section: Chapter Questions
Problem 23E: Summarize the relationship between pKa and acid strength by completing the following sentences: a....
Related questions
Question
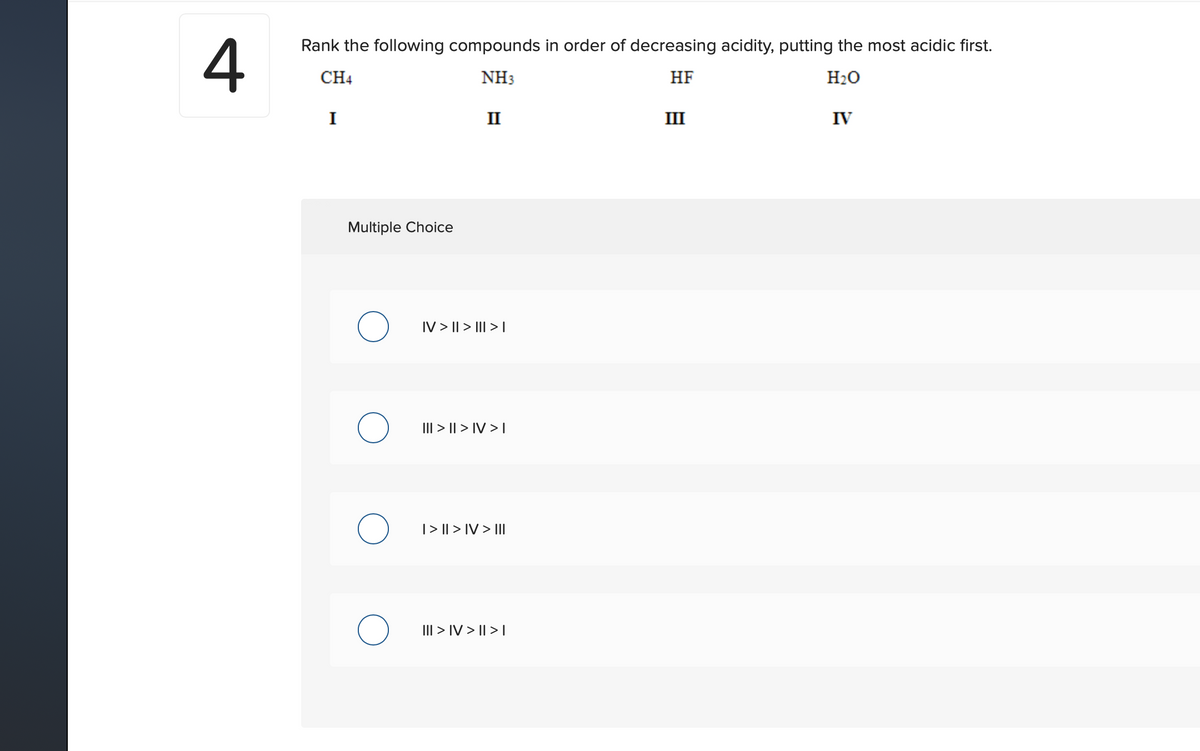
Transcribed Image Text:4
Rank the following compounds in order of decreasing acidity, putting the most acidic first.
CH4
NH3
HF
H20
I
II
III
IV
Multiple Choice
IV > || > II| > L
III > || > IV > T
|> || > IV > II
III > IV > || > |
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning