Rank the following elements according to their ionization energy. element ionization energy beryllium (Choose one) v potassium (Choose one) ♥ calcium (Choose one) ♥ francium (Choose one) ♥ Explanation Check lenou aiiy Huddle WebEx at 3pm (dia ECTI COL :1-650-479-3208 · Access Code (meeting
Rank the following elements according to their ionization energy. element ionization energy beryllium (Choose one) v potassium (Choose one) ♥ calcium (Choose one) ♥ francium (Choose one) ♥ Explanation Check lenou aiiy Huddle WebEx at 3pm (dia ECTI COL :1-650-479-3208 · Access Code (meeting
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 55AP: The outermost electron in an alkali-metal atom is sometimes described as resembling an electron in...
Related questions
Question
100%
I don’t know why but that’s the second answer from the similar question you guys answer wrong the ionization range was wrong please make sure this one is right
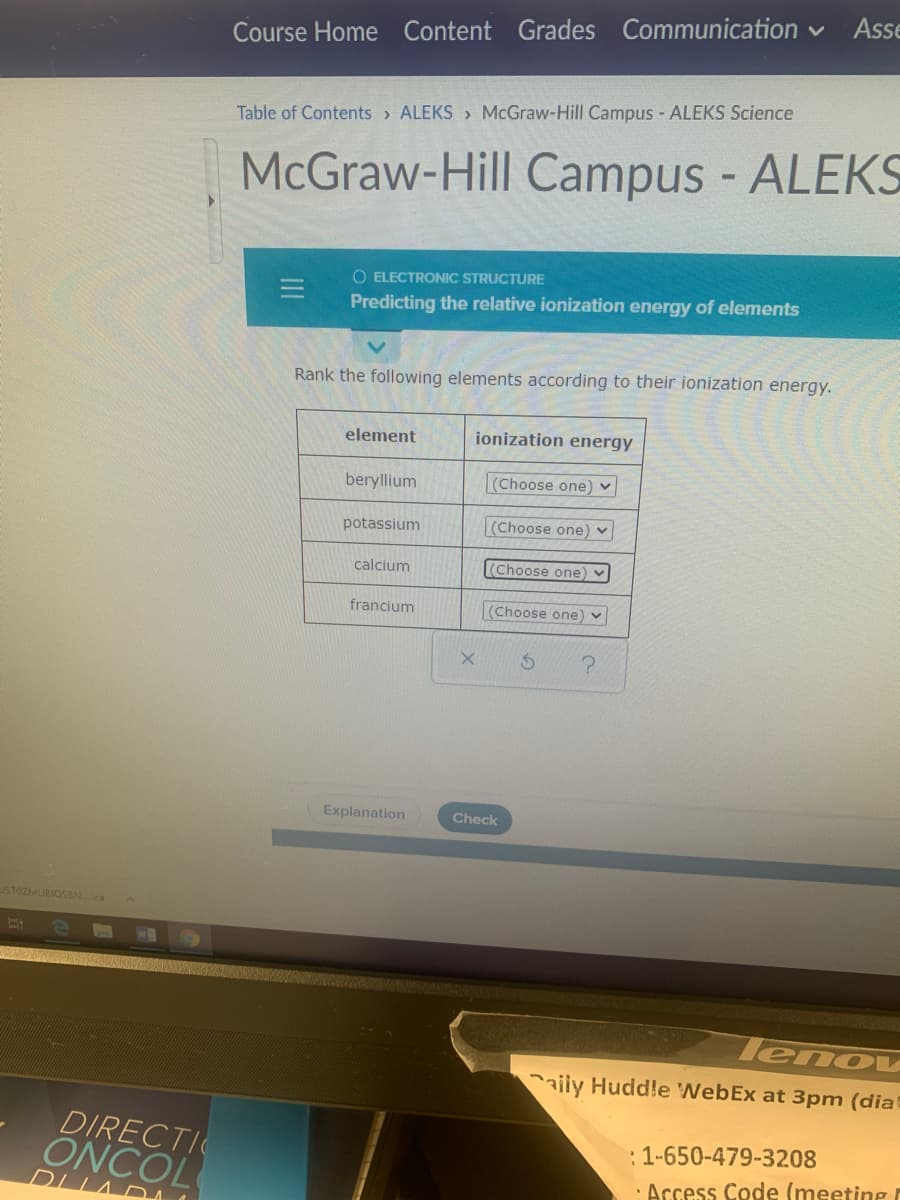
Transcribed Image Text:Asse
Course Home Content Grades Communication
Table of Contents > ALEKS > McGraw-Hill Campus - ALEKS Science
McGraw-Hill Campus - ALEKS
O ELECTRONIC STRUCTURE
Predicting the relative ionization energy of elements
Rank the following elements according to their ionization energy.
element
ionization energy
beryllium
(Choose one)
potassium
(Choose one) ♥
calcium
(Choose one
francium
(Choose one) v
Explanation
Check
STOZMUBIQSBN...ca
lenov
aily Huddle WebEx at 3pm (dia
DIRECTI
: 1-650-479-3208
ÔNCOL
DLLA DA
· Access Code (meeting
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning