CHE 170 (Mayo) Fall 2019 Review I Cor Arrange the following elements from greatest to least tendency to accept an electron. Rank from greatest to least tendency to accept an electron. To rank items as equivalent, overlap them. View Available Hint(s) Reset Help Least tendency Greatest tendency Na Mg Si The correct ranking cannot be determined. Previous Answers Submit Incorrect; Try Again; 7 attempts remaining ヘロの Provide Feedback Et DELL PADVANTAGE M OUCATION LOAN 20276 wwww Loa 0e g X
CHE 170 (Mayo) Fall 2019 Review I Cor Arrange the following elements from greatest to least tendency to accept an electron. Rank from greatest to least tendency to accept an electron. To rank items as equivalent, overlap them. View Available Hint(s) Reset Help Least tendency Greatest tendency Na Mg Si The correct ranking cannot be determined. Previous Answers Submit Incorrect; Try Again; 7 attempts remaining ヘロの Provide Feedback Et DELL PADVANTAGE M OUCATION LOAN 20276 wwww Loa 0e g X
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter2: Atomic Structure And Periodicity
Section: Chapter Questions
Problem 11ALQ: Consider the following statement "The ionization energy for the potassium atom is negative, because...
Related questions
Question
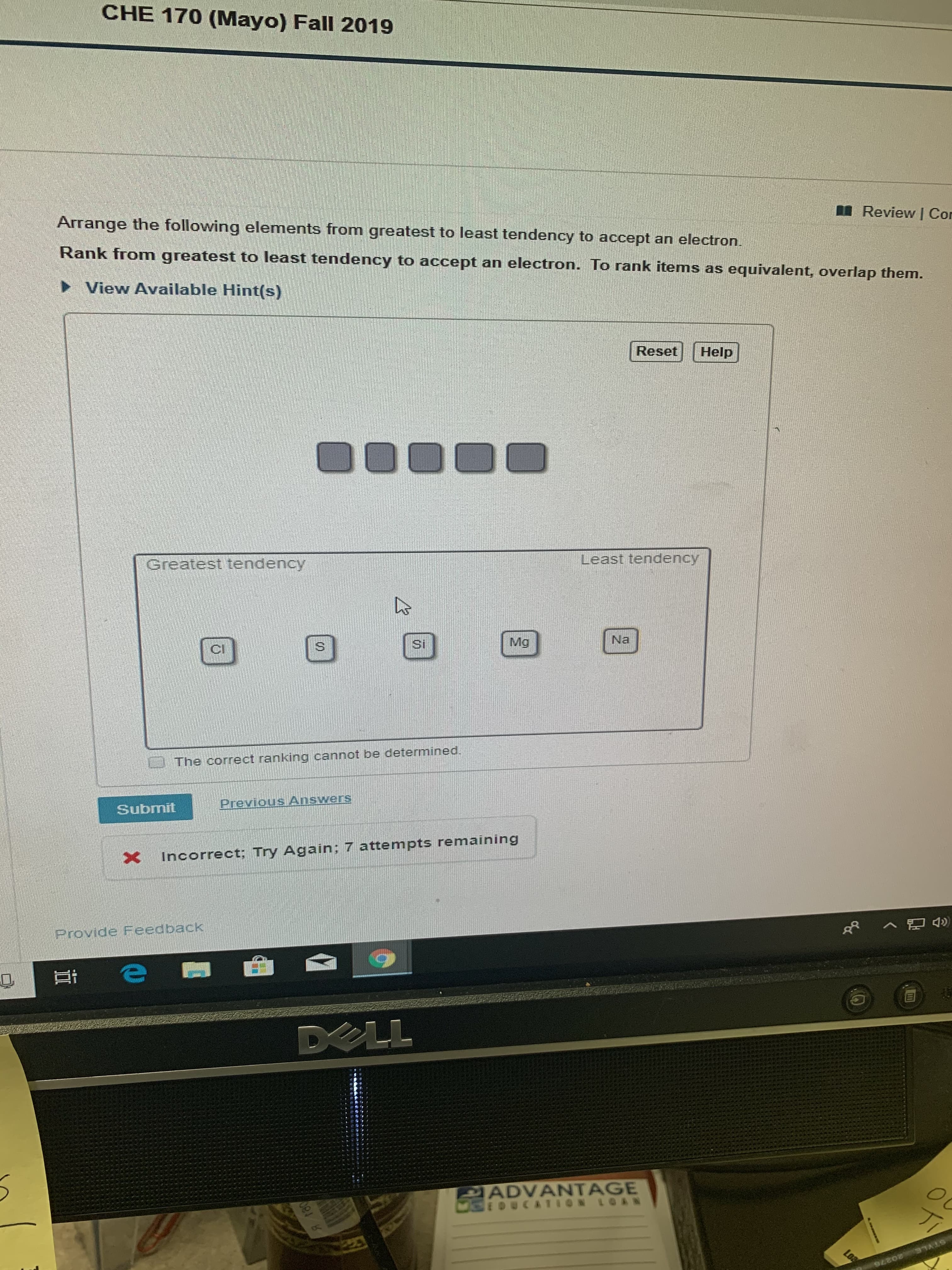
Transcribed Image Text:CHE 170 (Mayo) Fall 2019
Review I Cor
Arrange the following elements from greatest to least tendency to accept an electron.
Rank from
greatest to least tendency to accept an electron. To rank items
as equivalent, overlap them.
View Available Hint(s)
Reset
Help
Least tendency
Greatest tendency
Na
Mg
Si
The correct ranking cannot be determined.
Previous Answers
Submit
Incorrect; Try Again; 7 attempts remaining
ヘロの
Provide Feedback
Et
DELL
PADVANTAGE
M OUCATION LOAN
20276
wwww
Loa
0e g
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning