Reaction 15 min. (Ph),P. (Ph)sP=0 r.t. Procedure 1. Place 1 mmol of benzaldehyde and 1 mmol of (carbethoxymethylene)triphenylphosphorane in a mortar. Using a pestle, blend the two starting materials together until they form a homogeneous, translucent paste. 2. Allow the reaction mixture to stand for 15 min. Workup 3. Add 4 ml of hexane mixture (hexanes) to the mortar, scrape well using a spatula in order to dislodge product and co-product from the mortar and transfer the slurry you obtained into a Büchner funnel and vacuum filter. Rinse the mortal with an additional 3 ml of hexanes and wash the filter cake with this solvent. Isolation 4. Transfer the filtrate into a pre-weighed round bottom flask. Prepare two TLC plates using some of the fil- trate to spot your product, also spot benzaldehyde, benzoic acid and a solution of the material retained on the filter paper. To find the proper solvent to dissolve the latter, consult the Handbook of Chemistry and Physics (in Chem 20). Run two TLC plates. Observe the plates under UV-light, then expose one to iodine vapors, the other to a potassium permanganate solution. Report all observations in your notebook. 5. Remove the solvent using a rota-vap and determine percent yield.
Reaction 15 min. (Ph),P. (Ph)sP=0 r.t. Procedure 1. Place 1 mmol of benzaldehyde and 1 mmol of (carbethoxymethylene)triphenylphosphorane in a mortar. Using a pestle, blend the two starting materials together until they form a homogeneous, translucent paste. 2. Allow the reaction mixture to stand for 15 min. Workup 3. Add 4 ml of hexane mixture (hexanes) to the mortar, scrape well using a spatula in order to dislodge product and co-product from the mortar and transfer the slurry you obtained into a Büchner funnel and vacuum filter. Rinse the mortal with an additional 3 ml of hexanes and wash the filter cake with this solvent. Isolation 4. Transfer the filtrate into a pre-weighed round bottom flask. Prepare two TLC plates using some of the fil- trate to spot your product, also spot benzaldehyde, benzoic acid and a solution of the material retained on the filter paper. To find the proper solvent to dissolve the latter, consult the Handbook of Chemistry and Physics (in Chem 20). Run two TLC plates. Observe the plates under UV-light, then expose one to iodine vapors, the other to a potassium permanganate solution. Report all observations in your notebook. 5. Remove the solvent using a rota-vap and determine percent yield.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 1QAP: hat do the coefficients of a balanced chemical equation tell us about the proportions in which atoms...
Related questions
Question
comment on the greeness of the experiment. the percent yield was 25%, the atom economy 38.8%, and the reaction effieciency 9.7 %

Transcribed Image Text:3. Comment on the green chemistry of this experiment. That is, the nature and amount of
solvent (for example water, etc.), and atom economy (number of atoms in the reactants
versus products) in this experiment.
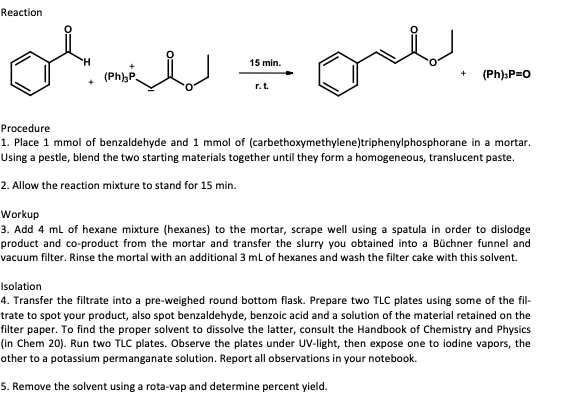
Transcribed Image Text:Reaction
onl
15 min.
(Ph),P.
(Ph)sP=0
r.t.
Procedure
1. Place 1 mmol of benzaldehyde and 1 mmol of (carbethoxymethylene)triphenylphosphorane in a mortar.
Using a pestle, blend the two starting materials together until they form a homogeneous, translucent paste.
2. Allow the reaction mixture to stand for 15 min.
Workup
3. Add 4 ml of hexane mixture (hexanes) to the mortar, scrape well using a spatula in order to dislodge
product and co-product from the mortar and transfer the slurry you obtained into a Büchner funnel and
vacuum filter. Rinse the mortal with an additional 3 ml of hexanes and wash the filter cake with this solvent.
Isolation
4. Transfer the filtrate into a pre-weighed round bottom flask. Prepare two TLC plates using some of the fil-
trate to spot your product, also spot benzaldehyde, benzoic acid and a solution of the material retained on the
filter paper. To find the proper solvent to dissolve the latter, consult the Handbook of Chemistry and Physics
(in Chem 20). Run two TLC plates. Observe the plates under UV-light, then expose one to iodine vapors, the
other to a potassium permanganate solution. Report all observations in your notebook.
5. Remove the solvent using a rota-vap and determine percent yield.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning