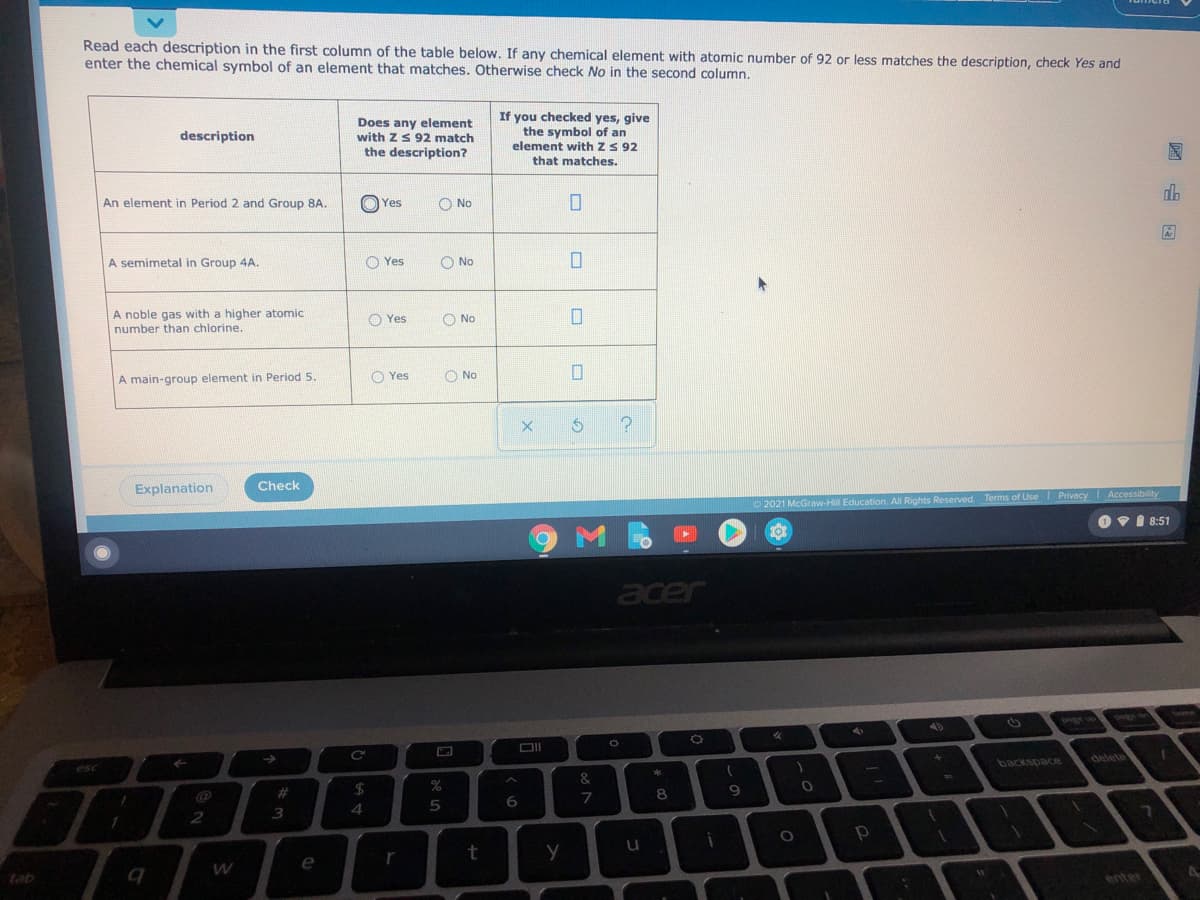
Read each description in the first column of the table below. If any chemical element with atomic number of 92 or less matches the description, check Yes and enter the chemical symbol of an element that matches. Otherwise check No in the second column. Does any element with zS 92 match the description? If you checked yes, give the symbol of an element with zS 92 description that matches. An element in Period 2 and Group 8A. O Yes O No A semimetal in Group 4A. O Yes O No A noble gas with a higher atomic number than chlorine. O Yes O No A main-group element in Period 5. O Yes O No
Atomic Number (Z) 92 used for Uranium. For the above mentioned condition in question we have choose elements with equal to or less than atomic number of 92.
(1) An element in period second and group 8A -
In period second Li, Be, B, C, N, O, F, Ne are present. In these elements 8A group element is Ne, Neon. It has atomic number of 10.
(2) A semimetal in group 4A -
In group 4A , the semimetal is Si, silicone. 4A group contains Carbon, silicone, germanium,tin ,lead etc in which silicone is semimetal. Silicone has atomic number of 14.
(3) A Noble gas with a higher atomic number than Chlorine -
Chlorine has atomic number of 17. Present in halogen group and period third. Argon ,At, with, atomic number 18 is higher Noble gas element than Chlorine.
(4) A main group element in period 5 -
s and p group elements are main group elements. In period 5, Rb, Sr, In, Sn, Sb, Te, I, and Xe are present.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps









