Read the descriptions of physical or chemical changes in the table below. Then decide whether the change will be spontaneous, if you can. Change During an endothermic chemical reaction, four moles of gaseous reactants are turned into two moles of gaseous products. A solid precipitates from a solution, releasing heat as it does so. A solid absorbs heat and turns to a gas. Is this change spontaneous? Yes. O No. O Can't decide with information given. Yes. O No. O Can't decide with information given. O Yes. O No. O Can't decide with information given.
Read the descriptions of physical or chemical changes in the table below. Then decide whether the change will be spontaneous, if you can. Change During an endothermic chemical reaction, four moles of gaseous reactants are turned into two moles of gaseous products. A solid precipitates from a solution, releasing heat as it does so. A solid absorbs heat and turns to a gas. Is this change spontaneous? Yes. O No. O Can't decide with information given. Yes. O No. O Can't decide with information given. O Yes. O No. O Can't decide with information given.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter16: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 2RQ: What is the second law of thermodynamics? For any process, there are four possible sign combinations...
Related questions
Question
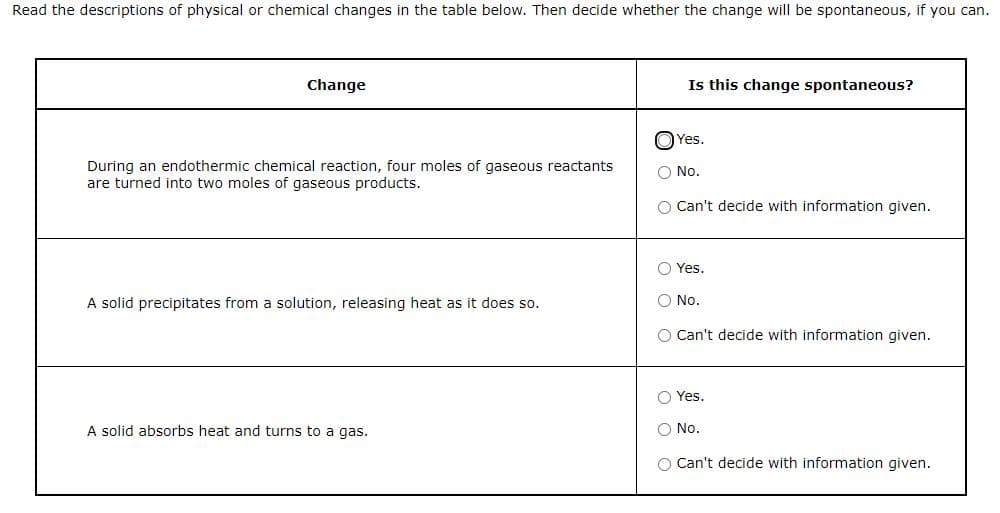
Transcribed Image Text:Read the descriptions of physical or chemical changes in the table below. Then decide whether the change will be spontaneous, if you can.
Change
During an endothermic chemical reaction, four moles of gaseous reactants
are turned into two moles of gaseous products.
A solid precipitates from a solution, releasing heat as it does so.
A solid absorbs heat and turns to a gas.
Is this change spontaneous?
Yes.
O No.
O Can't decide with information given.
O Yes.
O No.
O Can't decide with information given.
O Yes.
O No.
O Can't decide with information given.
Expert Solution
Step 1
Thermodynamics is branch of chemistry in which we deal with amount of heat evolved or absorbed by the system. In the given question it has been asked about spontaneity of the reaction.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning