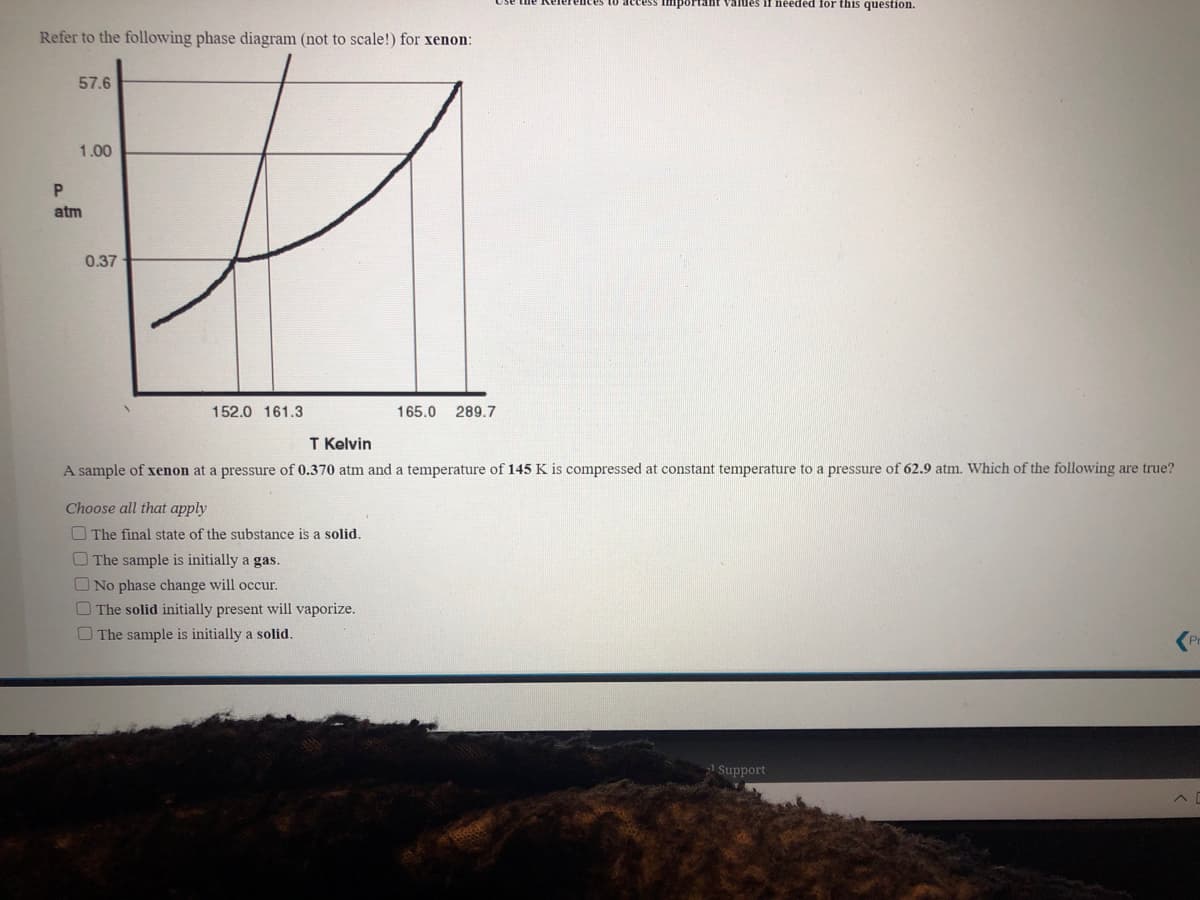
Refer to the following phase diagram (not to scale!) for xenon: 57.6 1.00 atm 0.37 152.0 161.3 165.0 289.7 T Kelvin A sample of xenon at a pressure of 0.370 atm and a temperature of 145 K is compressed at constant temperature to a pressure of 62.9 atm. Which of the following are true? Choose all that apply O The final state of the substance is a solid. O The sample is initially a gas. ONo phase change will occur. The solid initially present will vaporize. O The sample is initially a solid.
Refer to the following phase diagram (not to scale!) for xenon: 57.6 1.00 atm 0.37 152.0 161.3 165.0 289.7 T Kelvin A sample of xenon at a pressure of 0.370 atm and a temperature of 145 K is compressed at constant temperature to a pressure of 62.9 atm. Which of the following are true? Choose all that apply O The final state of the substance is a solid. O The sample is initially a gas. ONo phase change will occur. The solid initially present will vaporize. O The sample is initially a solid.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter10: Solids, Liquids, And Phase Transitions
Section: Chapter Questions
Problem 34P
Related questions
Question
Transcribed Image Text:ortant Values if needed for this question.
Refer to the following phase diagram (not to scale!) for xenon:
57.6
1.00
atm
0.37
152.0 161.3
165.0
289.7
T Kelvin
A sample of xenon at a pressure of 0.370 atm and a temperature of 145 K is compressed at constant temperature to a pressure of 62.9 atm. Which of the following are true?
Choose all that apply
O The final state of the substance is a solid.
OThe sample is initially a gas.
O No phase change will occur.
O The solid initially present will vaporize.
O The sample is initially a solid.
2 Support
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning