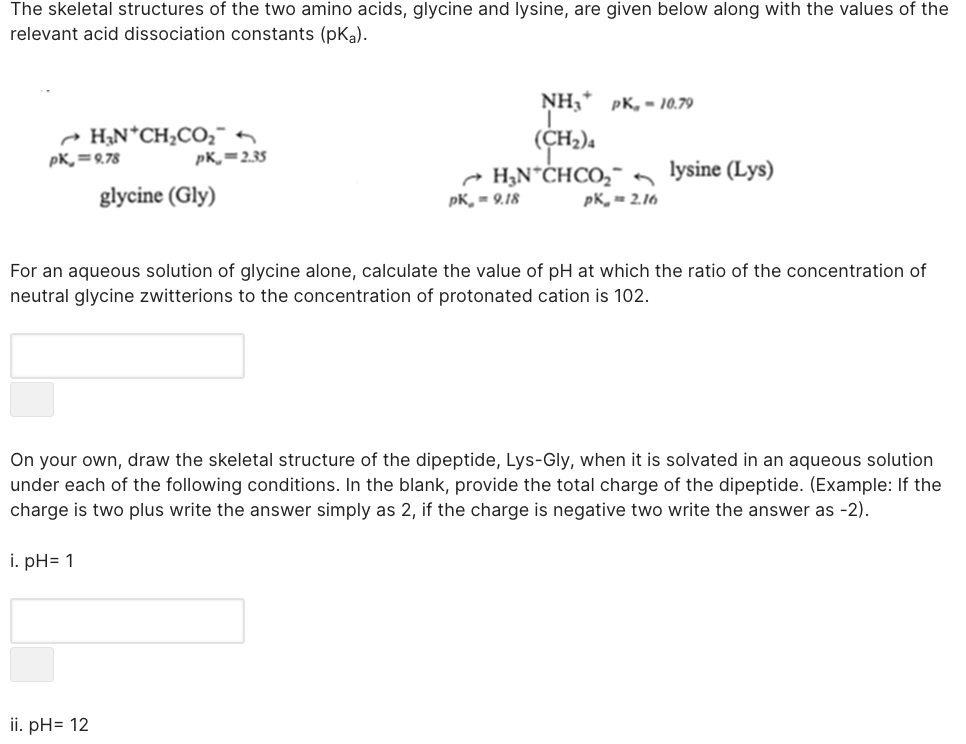
The skeletal structures of the two amino acids, glycine and lysine, are given below along with the values of the relevant acid dissociation constants (pKa). NH,* pK, - 10.79 - HạN*CH2CO,¯ pK,=9.78 (CH2)4 pK,=2.35 - H,N*ĊHCO,- lysine (Lys) glycine (Gly) pK, - 9.18 pK, - 2.16 For an aqueous solution of glycine alone, calculate the value of pH at which the ratio of the concentration of neutral glycine zwitterions to the concentration of protonated cation is 102.
Nucleotides
It is an organic molecule made up of three basic components- a nitrogenous base, phosphate,and pentose sugar. The nucleotides are important for metabolic reactions andthe formation of DNA (deoxyribonucleic acid) and RNA (ribonucleic acid).
Nucleic Acids
Nucleic acids are essential biomolecules present in prokaryotic and eukaryotic cells and viruses. They carry the genetic information for the synthesis of proteins and cellular replication. The nucleic acids are of two types: deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). The structure of all proteins and ultimately every biomolecule and cellular component is a product of information encoded in the sequence of nucleic acids. Parts of a DNA molecule containing the information needed to synthesize a protein or an RNA are genes. Nucleic acids can store and transmit genetic information from one generation to the next, fundamental to any life form.

Step by step
Solved in 2 steps with 3 images









