References Use the References to access important values if needed for this question. A sample of hydrogen gas has a density of g/L at a pressure of 0.602 atm and a temperature of 44 C. Assume ideal behavior. https://als-asp.cengage.info/als-asp/take FLV Type here to search Cip delete home t12 f10 prt sc 144 144 + backspace $4 7 80 00 %24
References Use the References to access important values if needed for this question. A sample of hydrogen gas has a density of g/L at a pressure of 0.602 atm and a temperature of 44 C. Assume ideal behavior. https://als-asp.cengage.info/als-asp/take FLV Type here to search Cip delete home t12 f10 prt sc 144 144 + backspace $4 7 80 00 %24
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter1: Gases And The Zeroth Law Of Thermodynamics
Section: Chapter Questions
Problem 1.21E: Pressures of gases in mixtures are referred to as partial pressures and are additive. 1.00 L of He...
Related questions
Question
Transcribed Image Text:References
Use the References to access important values if needed for this question.
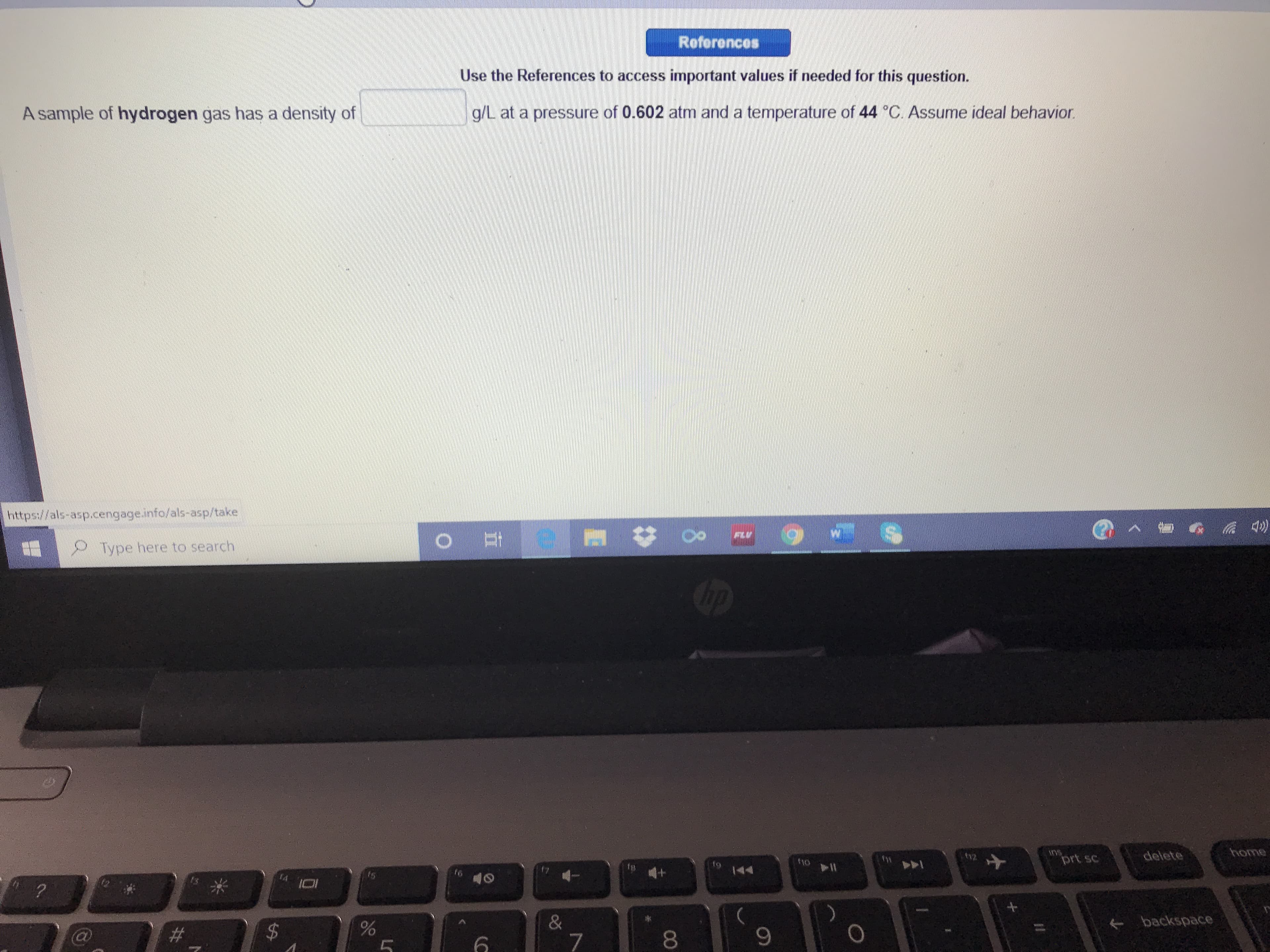
A sample of hydrogen gas has a density of
g/L at a pressure of 0.602 atm and a temperature of 44 C. Assume ideal behavior.
https://als-asp.cengage.info/als-asp/take
FLV
Type here to search
Cip
delete
home
t12
f10
prt sc
144
144
+ backspace
$4
7
80
00
%24
Expert Solution
Step 1
Use ideal gas equation:

Step 2
Given,
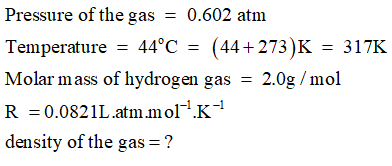
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning