Resonance structures for the "allyl carbanion" are shown below (I and II). CH CH=CH2 CH=CH-CH2 I II Which of the following statements regarding I and Il are correct? Select one: O A. The energy of the actual allyl carbanion is higher than the energy expected for resonance structuresI and II. B. Resonance structures I and II are nonequivalent resonance structures. C. The actual structure of an allyl carbanion is an average of structures I and II, with both resonance structures weighted equally. D. In the actual allyl carbanion, both C,C bonds are unequal in length.
Resonance structures for the "allyl carbanion" are shown below (I and II). CH CH=CH2 CH=CH-CH2 I II Which of the following statements regarding I and Il are correct? Select one: O A. The energy of the actual allyl carbanion is higher than the energy expected for resonance structuresI and II. B. Resonance structures I and II are nonequivalent resonance structures. C. The actual structure of an allyl carbanion is an average of structures I and II, with both resonance structures weighted equally. D. In the actual allyl carbanion, both C,C bonds are unequal in length.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter5: Resonance
Section: Chapter Questions
Problem 19CTQ: The C=O double bond is called a “carbonyl bond.” Acetone and othercarbonyl compounds are introduced...
Related questions
Question
HW4
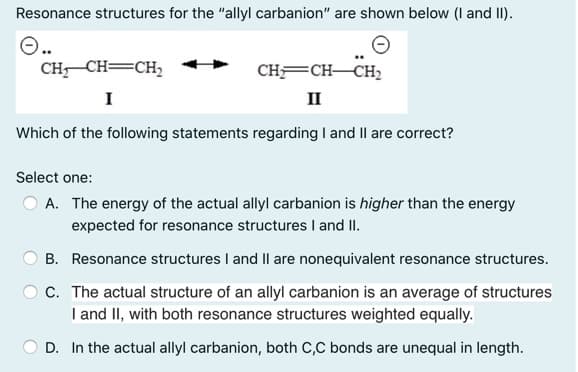
Transcribed Image Text:Resonance structures for the "allyl carbanion" are shown below (I and II).
CH CH=CH,
CHFCH-CH2
I
II
Which of the following statements regarding I and Il are correct?
Select one:
O A. The energy of the actual allyl carbanion is higher than the energy
expected for resonance structures I and II.
B. Resonance structures I and II are nonequivalent resonance structures.
C. The actual structure of an allyl carbanion is an average of structures
I and II, with both resonance structures weighted equally.
D. In the actual allyl carbanion, both C,C bonds are unequal in length.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
