Results of Firing Alpha Particles at Gold Foil Observation Proportion: Alpha particles went straight through gold foil > 98% Alpha particles went through gold foil but were deflected at large angles = 2% Alpha particles bounced off gold foil = 0.01% 9. What information do the experimental results above reveal about the nucleus of the gold atom? (A) The nucleus contains less than half the mass of the atom. (B) The nucleus is small and is the densest part of the atom. (C) The nucleus contains small positive and negative particles. (D) The nucleus is large and occupies most of the atom's space.
Results of Firing Alpha Particles at Gold Foil Observation Proportion: Alpha particles went straight through gold foil > 98% Alpha particles went through gold foil but were deflected at large angles = 2% Alpha particles bounced off gold foil = 0.01% 9. What information do the experimental results above reveal about the nucleus of the gold atom? (A) The nucleus contains less than half the mass of the atom. (B) The nucleus is small and is the densest part of the atom. (C) The nucleus contains small positive and negative particles. (D) The nucleus is large and occupies most of the atom's space.
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter19: Radioactivity And Nuclear Energy
Section: Chapter Questions
Problem 11A
Related questions
Question
100%
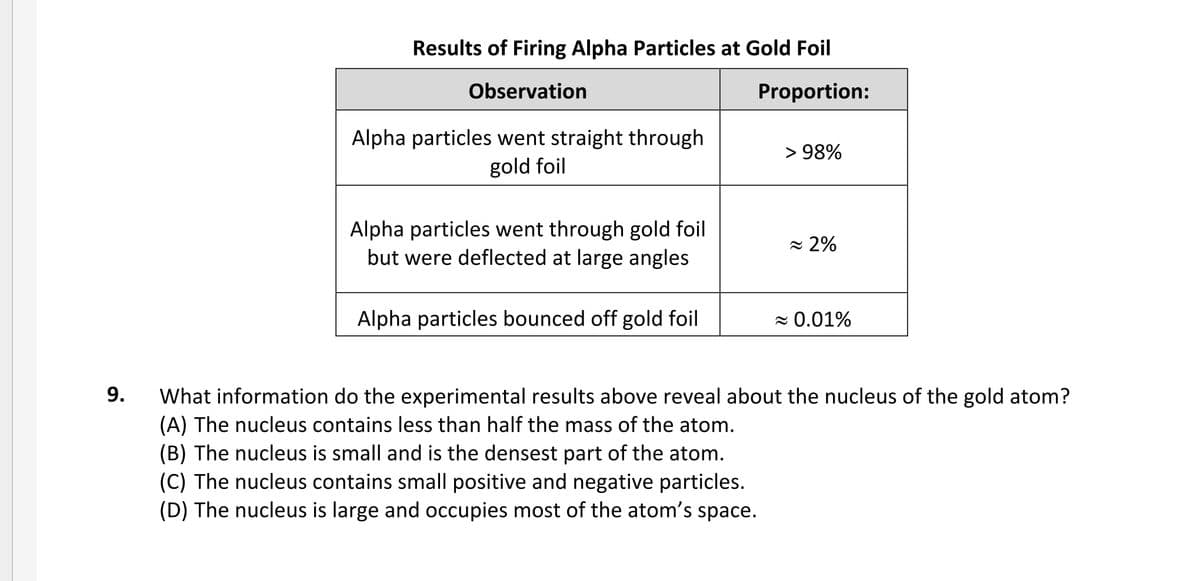
Transcribed Image Text:Results of Firing Alpha Particles at Gold Foil
Observation
Proportion:
Alpha particles went straight through
gold foil
> 98%
Alpha particles went through gold foil
but were deflected at large angles
= 2%
Alpha particles bounced off gold foil
z 0.01%
9.
What information do the experimental results above reveal about the nucleus of the gold atom?
(A) The nucleus contains less than half the mass of the atom.
(B) The nucleus is small and is the densest part of the atom.
(C) The nucleus contains small positive and negative particles.
(D) The nucleus is large and occupies most of the atom's space.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning