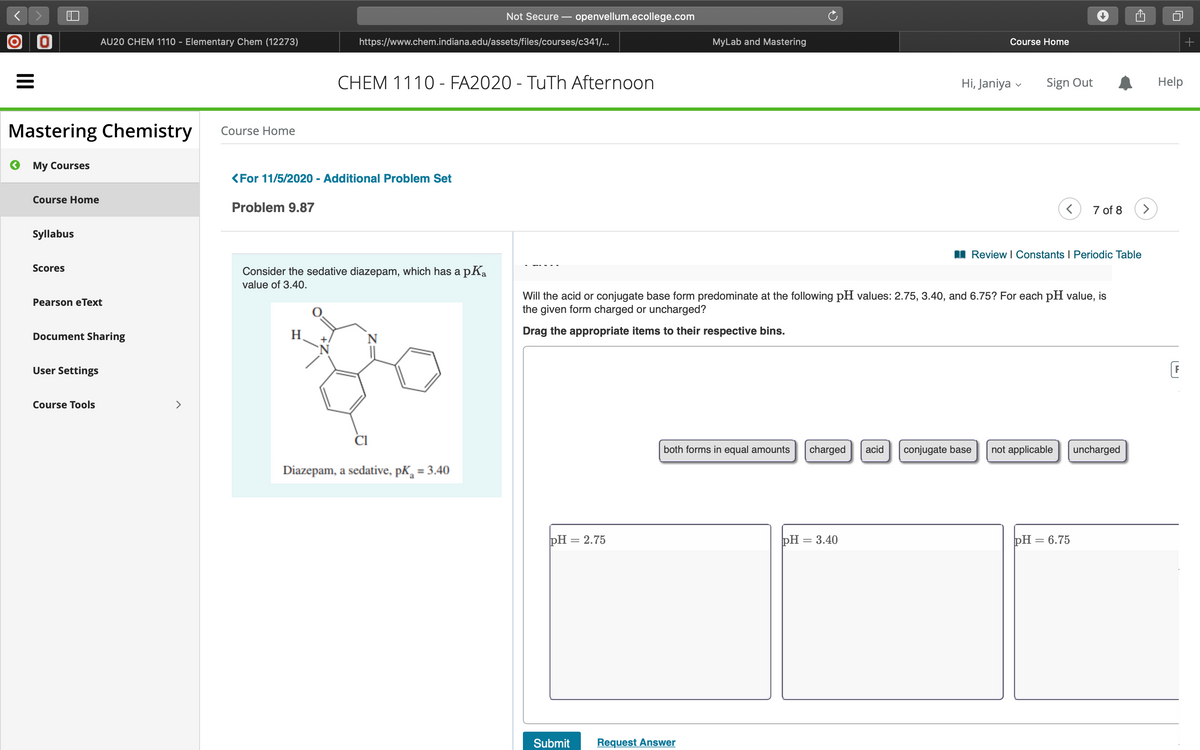
Review I Constants I Periodic Table Consider the sedative diazepam, which has a pK, value of 3.40. Will the acid or conjugate base form predominate at the following pH values: 2.75, 3.40, and 6.75? For each pH value, is the given form charged or uncharged? H. Drag the appropriate items to their respective bins. both forms in equal amounts charged conjugate base not applicable uncharged acid Diazepam, a sedative, PK, = 3.40
Review I Constants I Periodic Table Consider the sedative diazepam, which has a pK, value of 3.40. Will the acid or conjugate base form predominate at the following pH values: 2.75, 3.40, and 6.75? For each pH value, is the given form charged or uncharged? H. Drag the appropriate items to their respective bins. both forms in equal amounts charged conjugate base not applicable uncharged acid Diazepam, a sedative, PK, = 3.40
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.9QAP
Related questions
Question
I'm very confused on how to do these
Transcribed Image Text:Not Secure – openvellum.ecollege.com
O O
AU20 CHEM 1110 - Elementary Chem (12273)
https://www.chem.indiana.edu/assets/files/courses/c341/...
MyLab and Mastering
Course Home
CHEM 1110 - FA2020 - TuTh Afternoon
Hi, Janiya
Sign Out
Help
Mastering Chemistry
Course Home
O My Courses
<For 11/5/2020 - Additional Problem Set
Course Home
Problem 9.87
7 of 8
>
Syllabus
I Review I Constants I Periodic Table
Scores
Consider the sedative diazepam, which has a pKa
value of 3.40.
Will the acid or conjugate base form predominate at the following pH values: 2.75, 3.40, and 6.75? For each pH value, is
the given form charged or uncharged?
Pearson eText
రెం
Document Sharing
H
N
Drag the appropriate items to their respective bins.
+.
User Settings
Course Tools
>
both forms in equal amounts
charged
acid
conjugate base
not applicable
uncharged
Diazepam, a sedative, pK, = 3.40
pH = 2.75
РН — 3.40
РH — 6.75
Submit
Request Answer
Transcribed Image Text:Not Secure – openvellum.ecollege.com
O O
AU20 CHEM 1110 - Elementary Chem (12273)
https://www.chem.indiana.edu/assets/files/courses/c341/...
MyLab and Mastering
Course Home
CHEM 1110 - FA2020 - TuTh Afternoon
Hi, Janiya
Sign Out
Help
Mastering Chemistry
Course Home
O My Courses
<For 11/5/2020 - Additional Problem Set
Course Home
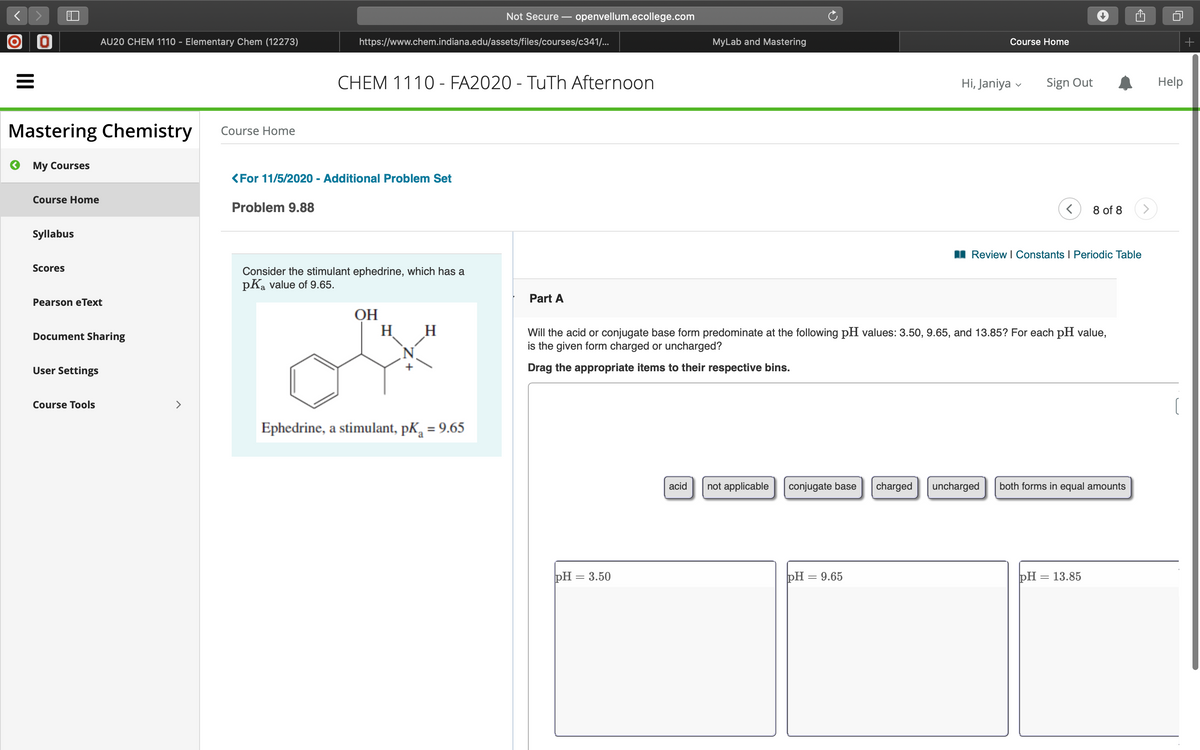
Problem 9.88
8 of 8
Syllabus
I Review I Constants I Periodic Table
Scores
Consider the stimulant ephedrine, which has a
pKa value of 9.65.
Part A
Pearson eText
ОН
H H
Will the acid or conjugate base form predominate at the following pH values: 3.50, 9.65, and 13.85? For each pH value,
is the given form charged or uncharged?
Document Sharing
User Settings
Drag the appropriate items to their respective bins.
Course Tools
>
Ephedrine, a stimulant, pK, = 9.65
acid
not applicable
conjugate base
charged
uncharged
both forms in equal amounts
pH = 3.50
pH = 9.65
pH = 13.85
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

