Problem Specialized cells in the stomach release HCl to aid digestion. If they release too much, the excess can be neutralized with an antacid. A common antacid contains magnesium hydroxide, which reacts with the acid to form water and magnesium chloride solution. As a government chemist testing commercial antacids, you use 0.10 M HCl to simulate the acid concentration in the stomach. How many liters of “stomach acid” react
with a tablet containing 0.10 g of magnesium hydroxide?
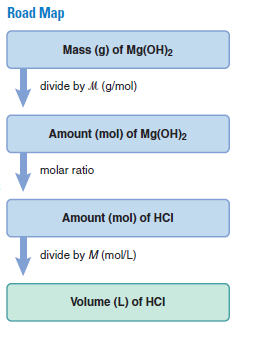
Plan We are given the mass (0.10 g) of magnesium hydroxide, Mg(OH)2, that reacts with the acid. We also know the acid concentration (0.10 M) and must find the acid volume. After writing the balanced equation, we convert the mass (g) of Mg(OH)2 to amount (mol) and use the molar ratio to find the amount (mol) of HCl that reacts with it. Then, we use the molarity of HCl to find the volume (L) that contains this amount (see the road map).
Trending now
This is a popular solution!
Step by step
Solved in 2 steps









