Running the reaction: 1.25 of the p-bromoacetanilide and 10 mL of water were placed in a 100 mL round- bottomed flask After chilling the mixture in an ice bath for 15 minutes, 10 mL of concentrated sulfuric acid was added slowly (dropwise with a Pasteur pipette) A condenser was attached and the mixture was refluxed for 60 minutes The solution was allowed to cool down to room temperature TLC using hexane/ethyl acetate (8:2) as mobile phase shows a large spot very close to the starting line (RF0.08) and a very faint spot about halfway up (R=0.45) corresponding to the anilide Product isolation: The mixture was poured into 20 mL of ice-cold water and then slowly neutralized with 10 M NaOH (a total of 19 mL to get the pH=8) After cooing the mixture in an ice bath, it was extracted three times with 10 mL dichloromethane (the organic layer was the bottom layer) The combined organic layers were extracted with 20 mL of water and 20 mL of saturated sodium chloride solution After carefully separating the layers, the organic layer was dried over a minimum amount of anhydrous sodium sulfate The solvent was allowed to evaporate leaving behind a white solid, which was allowed to dry Product characterization: 0.71 g of the product М.р.: 63.5-65.3 °C
Running the reaction: 1.25 of the p-bromoacetanilide and 10 mL of water were placed in a 100 mL round- bottomed flask After chilling the mixture in an ice bath for 15 minutes, 10 mL of concentrated sulfuric acid was added slowly (dropwise with a Pasteur pipette) A condenser was attached and the mixture was refluxed for 60 minutes The solution was allowed to cool down to room temperature TLC using hexane/ethyl acetate (8:2) as mobile phase shows a large spot very close to the starting line (RF0.08) and a very faint spot about halfway up (R=0.45) corresponding to the anilide Product isolation: The mixture was poured into 20 mL of ice-cold water and then slowly neutralized with 10 M NaOH (a total of 19 mL to get the pH=8) After cooing the mixture in an ice bath, it was extracted three times with 10 mL dichloromethane (the organic layer was the bottom layer) The combined organic layers were extracted with 20 mL of water and 20 mL of saturated sodium chloride solution After carefully separating the layers, the organic layer was dried over a minimum amount of anhydrous sodium sulfate The solvent was allowed to evaporate leaving behind a white solid, which was allowed to dry Product characterization: 0.71 g of the product М.р.: 63.5-65.3 °C
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter20: Dienes, Conjugated Systems, And Pericyclic Reactions
Section: Chapter Questions
Problem 20.27P
Related questions
Question
FIND THE PERCENTAGE YIELD, and include the moles of the reaction (part 3)

Transcribed Image Text:Part 3:
Running the reaction:
1.25 g of the p-bromoacetanilide and 10 mL of water were placed in a 100 mL round-
bottomed flask
After chilling the mixture in an ice bath for 15 minutes, 10 mL of concentrated sulfuric
acid was added slowly (dropwise with a Pasteur pipette)
A condenser was attached and the mixture was refluxed for 60 minutes
The solution was allowed to cool down to room temperature
TLC using hexane/ethyl acetate (8:2) as mobile phase shows a large spot very close to the
starting line (R=0.08) and a very faint spot about halfway up (RF0.45) corresponding to
the anilide
Product isolation:
The mixture was poured into 20 mL of ice-cold water and then slowly neutralized with
10 M NaOH (a total of 19 mL to get the pH=8)
After cooing the mixture in an ice bath, it was extracted three times with 10 mL
dichloromethane (the organic layer was the bottom layer)
The combined organic layers were extracted with 20 mL of water and 20 mL of saturated
sodium chloride solution
After carefully separating the layers, the organic layer was dried over a minimum amount
of anhydrous sodium sulfate
The solvent was allowed to evaporate leaving behind a white solid, which was allowed to
dry
Product characterization:
0.71 g of the product
М.p.: 63.5-65.3 °C
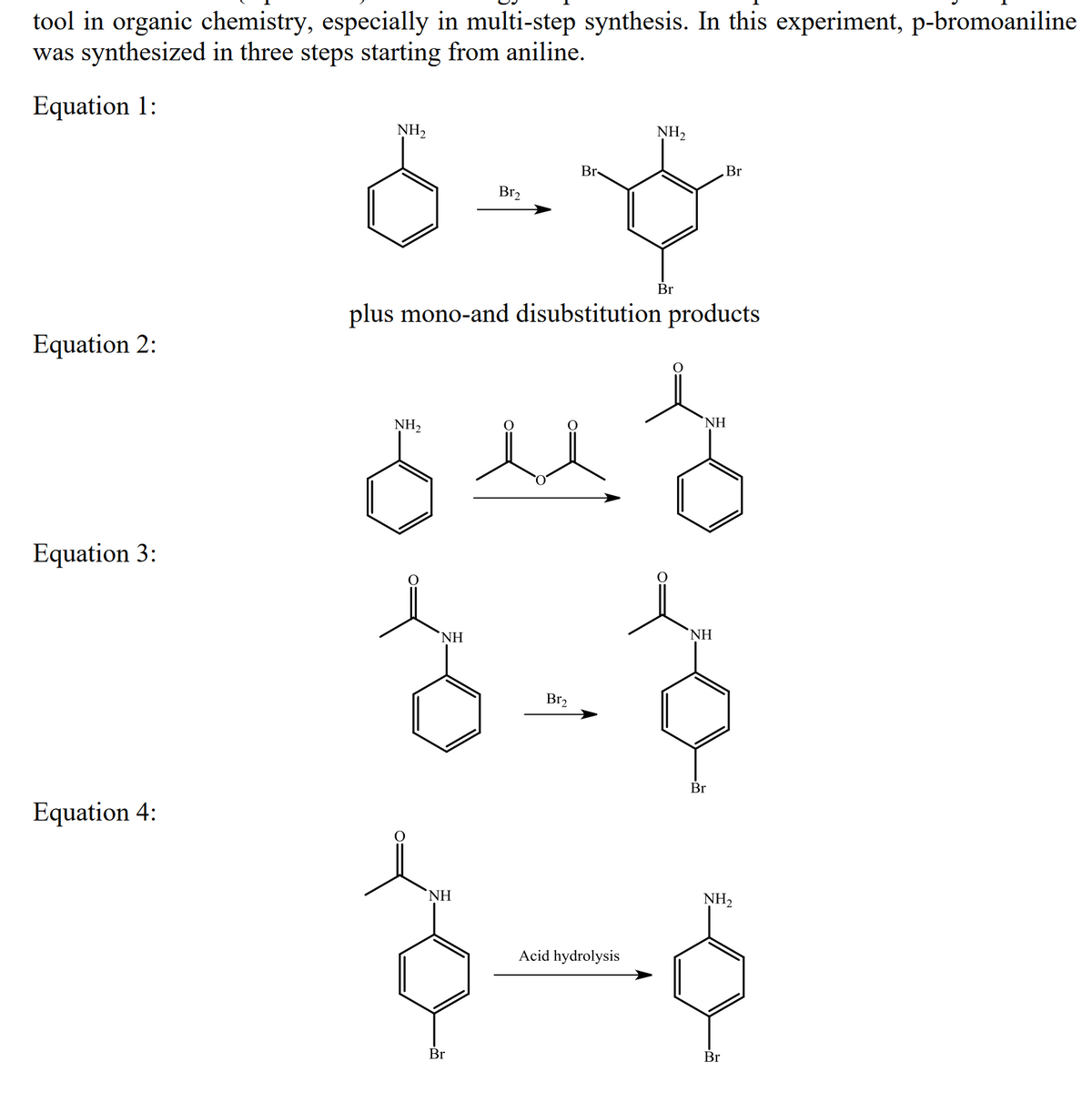
Transcribed Image Text:tool in organic chemistry, especially in multi-step synthesis. In this experiment, p-bromoaniline
was synthesized in three steps starting from aniline.
Equation 1:
NH2
NH,
Br-
Br
Br2
Br
plus mono-and disubstitution products
Equation 2:
NH2
NH
Equation 3:
`NH
NH
Br2
Br
Equation 4:
NH
NH2
Acid hydrolysis
Br
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning