Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter20: Chemistry Of Hydrogen, Elements In Group 3a Through 6a, And The Noble Gases
Section: Chapter Questions
Problem 20.47QE
Related questions
Question
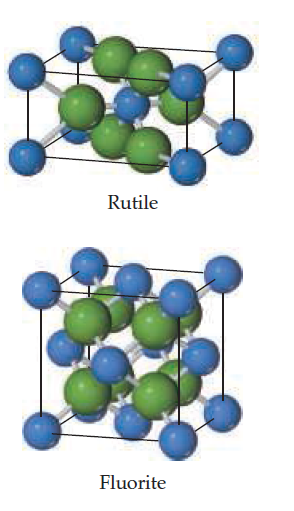
The rutile and fluorite structures, shown here (anions are
colored green), are two of the most common structure types
of ionic compounds where the cation to anion ratio is 1 : 2.
(a) For CaF2 and ZnF2 use ionic radii, Ca2+ 1r = 1.14 A° 2,
Zn2+ 1r = 0.88 A° 2, and F- 1r = 1.19 A° 2, to predict which
compound is more likely to crystallize with the fluorite
structure and which with the rutile structure. (b) What are
the coordination numbers of the cations and anions in each
of these structures?
Transcribed Image Text:Rutile
Fluorite
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning