Sample Brz in CCl (dark) Brz in CCl (light) KMNO4 in NaOH AGNO, in NH3 Fading of reddish- brown color 1 Fading of reddish- Brown precipitates Silver precipitates brown color 2 No reaction Fading of reddish- No reaction No reaction brown color 3 Fading of reddish- Fading of reddish- Brown precipitates No reaction brown color brown color H3C. CH3 „CH3 CH3 H;C H3C CH3 H3C Sample Identity and Rationalization 1 3
Sample Brz in CCl (dark) Brz in CCl (light) KMNO4 in NaOH AGNO, in NH3 Fading of reddish- brown color 1 Fading of reddish- Brown precipitates Silver precipitates brown color 2 No reaction Fading of reddish- No reaction No reaction brown color 3 Fading of reddish- Fading of reddish- Brown precipitates No reaction brown color brown color H3C. CH3 „CH3 CH3 H;C H3C CH3 H3C Sample Identity and Rationalization 1 3
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 103QRT: In Table 12.1 (←Sec. 12-3a) the equilibrium constant for the reaction
⇋
is given as 4.2 × 1052. If...
Related questions
Question
Based on the table, identify the identity of the samples. Choose the corresponding structure from the given choices. Explain how you arrived in the chosen structure.
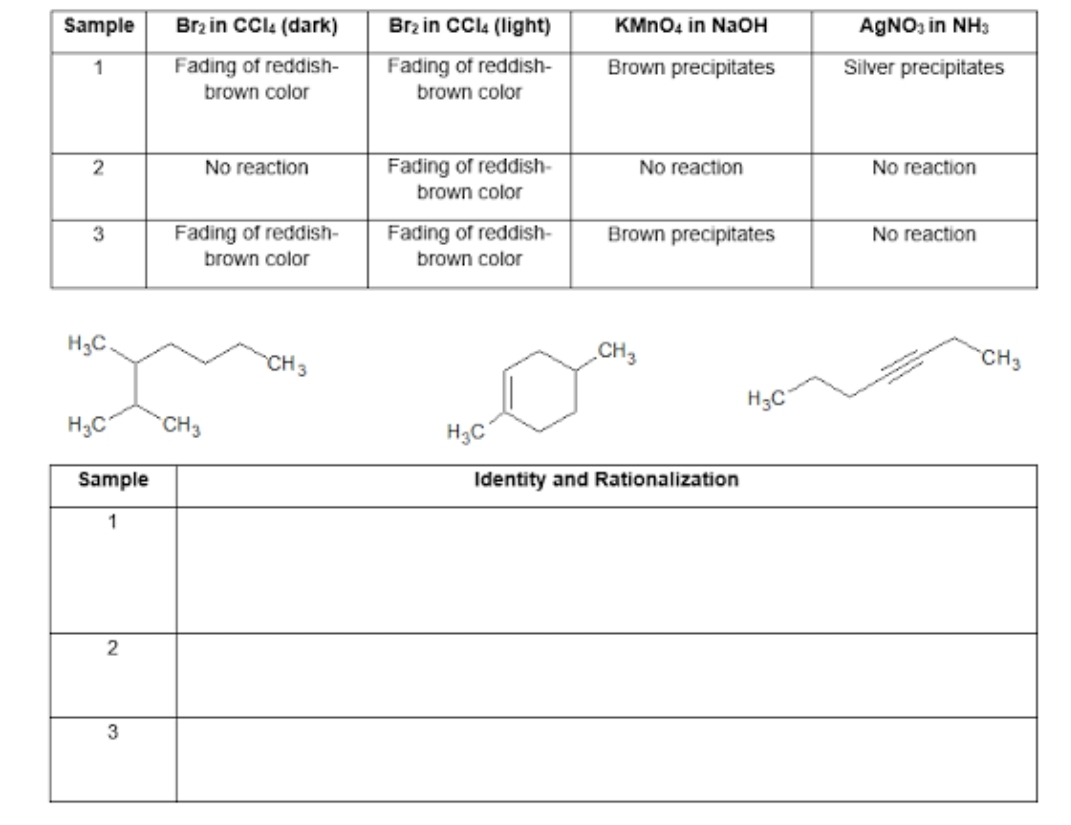
Transcribed Image Text:Sample
Brz in CCI4 (dark)
Brz in CCI (light)
KMNO4 in NaOH
AGNO, in NH3
Fading of reddish-
brown color
Fading of reddish-
brown color
1
Brown precipitates
Silver precipitates
2
No reaction
Fading of reddish-
No reaction
No reaction
brown color
Fading of reddish-
brown color
Fading of reddish-
brown color
3
Brown precipitates
No reaction
H3C.
CH3
CH3
CH3
H3C
H3C
CH3
H3C
Sample
Identity and Rationalization
1
3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning