Sapling Learning | Lenoir-Rhyne | X y! Yahoo - login acid or base calcul 17.3: Acid-Base Tit y! Calculate the pH o calculator - Googe b A 0.760g0.760 gsa https://www.saplinglearning.com/ibiscms/mod/flcn/view.php?id=11859017 Sapling Learning HW 14A Homeworki Leticia Venancio macmillan learning Sapling Learning > Lenoir-Rhyne University - CHE 104 - Spring20 - STEELE > Activities and Due Dates > HW 14A Homework#2 Titrations E 1 of 13 Questions く O Assignment Score: Lx Give Up? O Hint 73.4% Resources Check Answer 1 Question 0% Question 1 of 13 O of o Attempts A 0.328 g sample of a monoprotic acid is dissolved in water and titrated with 0.130 M KOH. What is the molar mass of 2 Question 0% O of o Attempts the acid if 28.0 mL of the KOH solution is required to neutralize the sample? -2 3 Question 0% 1 of o Attempts In Progress .7 molar mass: g/mol 10 11 O 4 Question 100% 12 13 1 of o Attempts Correct 15 16 17 --18 O 5 Question 99.3% 19 20 3 of o Attempts 21 Correct 22 23 24 O 6 Question 100% 1 of o Attempts Correct O7 Question 100% 1 of o Attempts Correct O8 Question 99% 2 of o Attempts Correct 9 Question 60% 1 of o Attempts In Progress © 2011-2020 Sapling Learning, Inc. about us privacy policy terms of use contact us help careers 3:26 PM O Type here to search 23 4/5/2020 K入 KY
Sapling Learning | Lenoir-Rhyne | X y! Yahoo - login acid or base calcul 17.3: Acid-Base Tit y! Calculate the pH o calculator - Googe b A 0.760g0.760 gsa https://www.saplinglearning.com/ibiscms/mod/flcn/view.php?id=11859017 Sapling Learning HW 14A Homeworki Leticia Venancio macmillan learning Sapling Learning > Lenoir-Rhyne University - CHE 104 - Spring20 - STEELE > Activities and Due Dates > HW 14A Homework#2 Titrations E 1 of 13 Questions く O Assignment Score: Lx Give Up? O Hint 73.4% Resources Check Answer 1 Question 0% Question 1 of 13 O of o Attempts A 0.328 g sample of a monoprotic acid is dissolved in water and titrated with 0.130 M KOH. What is the molar mass of 2 Question 0% O of o Attempts the acid if 28.0 mL of the KOH solution is required to neutralize the sample? -2 3 Question 0% 1 of o Attempts In Progress .7 molar mass: g/mol 10 11 O 4 Question 100% 12 13 1 of o Attempts Correct 15 16 17 --18 O 5 Question 99.3% 19 20 3 of o Attempts 21 Correct 22 23 24 O 6 Question 100% 1 of o Attempts Correct O7 Question 100% 1 of o Attempts Correct O8 Question 99% 2 of o Attempts Correct 9 Question 60% 1 of o Attempts In Progress © 2011-2020 Sapling Learning, Inc. about us privacy policy terms of use contact us help careers 3:26 PM O Type here to search 23 4/5/2020 K入 KY
Chapter14: Acids And Bases
Section: Chapter Questions
Problem 10RQ: For oxyacids, how does acid strength depend on a. the strength of the bond to the acidic hydrogen...
Related questions
Question
Transcribed Image Text:Sapling Learning |
Lenoir-Rhyne | X
y! Yahoo - login
acid or base calcul
17.3: Acid-Base Tit
y! Calculate the pH o
calculator - Googe b A 0.760g0.760 gsa
https://www.saplinglearning.com/ibiscms/mod/flcn/view.php?id=11859017
Sapling Learning
HW 14A Homeworki
Leticia Venancio
macmillan learning
Sapling Learning > Lenoir-Rhyne University - CHE 104 - Spring20 - STEELE > Activities and Due Dates > HW 14A Homework#2 Titrations
E 1 of 13 Questions
く
O Assignment Score:
Lx Give Up?
O Hint
73.4%
Resources
Check Answer
1 Question
0%
Question 1 of 13
O of o Attempts
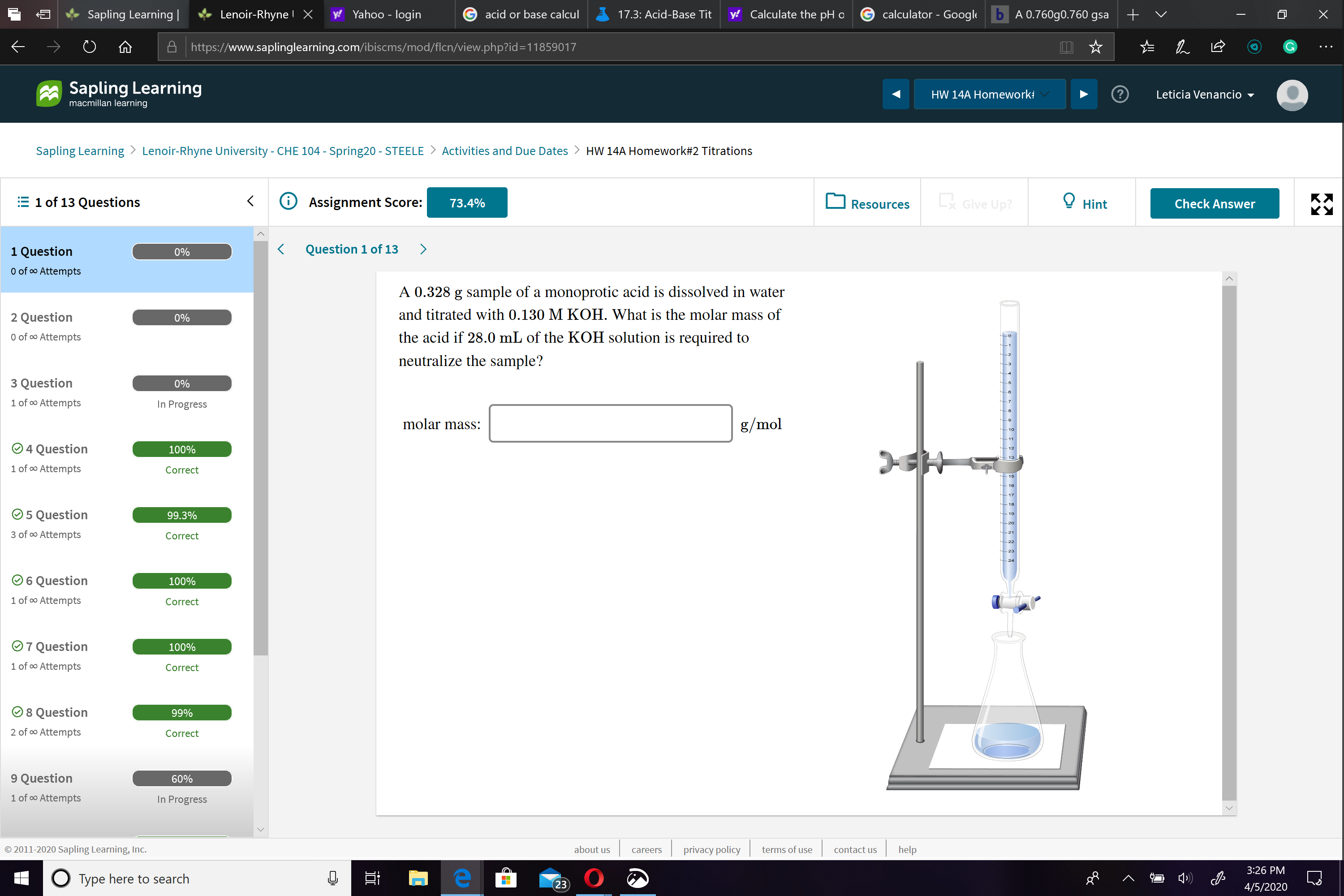
A 0.328 g sample of a monoprotic acid is dissolved in water
and titrated with 0.130 M KOH. What is the molar mass of
2 Question
0%
O of o Attempts
the acid if 28.0 mL of the KOH solution is required to
neutralize the sample?
-2
3 Question
0%
1 of o Attempts
In Progress
.7
molar mass:
g/mol
10
11
O 4 Question
100%
12
13
1 of o Attempts
Correct
15
16
17
--18
O 5 Question
99.3%
19
20
3 of o Attempts
21
Correct
22
23
24
O 6 Question
100%
1 of o Attempts
Correct
O7 Question
100%
1 of o Attempts
Correct
O8 Question
99%
2 of o Attempts
Correct
9 Question
60%
1 of o Attempts
In Progress
© 2011-2020 Sapling Learning, Inc.
about us
privacy policy
terms of use
contact us
help
careers
3:26 PM
O Type here to search
23
4/5/2020
K入
KY
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
