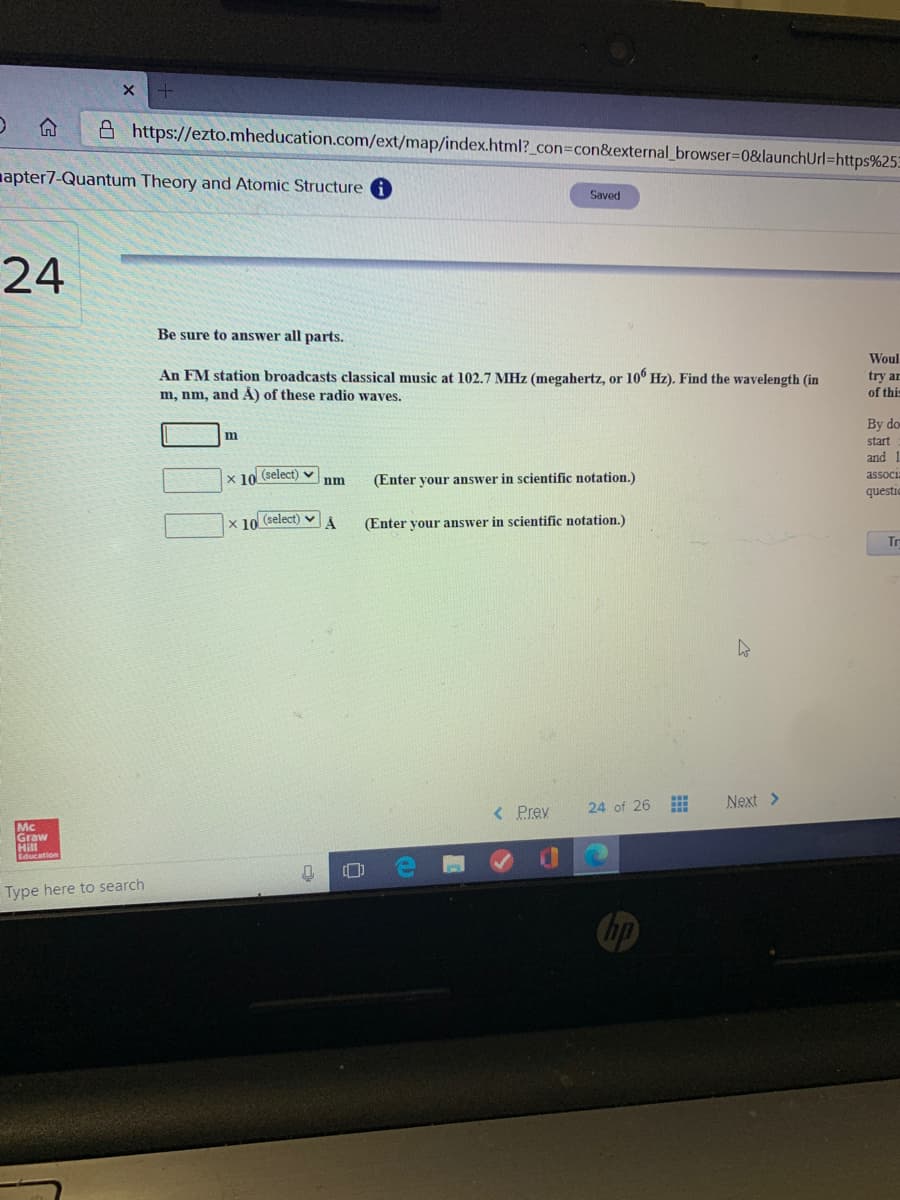
Saved 24 Be sure to answer all parts. Woul An FM station broadcasts classical music at 102.7 MHz (megahertz, or 10° Hz). Find the wavelength (in m, nm, and A) of these radio waves. try ar of this By do start and 1 x 10 (select) v associa nm (Enter your answer in scientific notation.) questic x 10 (select) v (Enter your answer in scientific notation.) Tr
Saved 24 Be sure to answer all parts. Woul An FM station broadcasts classical music at 102.7 MHz (megahertz, or 10° Hz). Find the wavelength (in m, nm, and A) of these radio waves. try ar of this By do start and 1 x 10 (select) v associa nm (Enter your answer in scientific notation.) questic x 10 (select) v (Enter your answer in scientific notation.) Tr
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter6: Electronic Structure And The Periodic Table
Section: Chapter Questions
Problem 7QAP: Lasers are now used for the total or partial removal of skin tattoos. A laser with a wavelenth of...
Related questions
Question
Transcribed Image Text:命
A https://ezto.mheducation.com/ext/map/index.html?_con%3Dcon&external_browser3D0&launchUrl=https%25:
napter7-Quantum Theory and Atomic Structure
Saved
24
Be sure to answer all parts.
An FM station broadcasts classical music at 102.7 MHz (megahertz, or 10° Hz). Find the wavelength (in
m, nm, and A) of these radio waves.
Woul
try ar
of this
By do
start
and 1
associ
X 10 (select) v
(Enter your answer in scientific notation.)
nm
questic
X 10 (select) v
(Enter your answer in scientific notation.)
Tr
< Prev
24 of 26
Next >
Mc
Graw
Hill
Type here to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning