View Available Hint(s) ΑΣφ ? E = kJ Submit • Part B visible light (490 nm) Express your answer using three significant figures. > View Available Hint(s) ΑΣφ E = kJ
View Available Hint(s) ΑΣφ ? E = kJ Submit • Part B visible light (490 nm) Express your answer using three significant figures. > View Available Hint(s) ΑΣφ E = kJ
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter20: Molecular Mass Spectrometry
Section: Chapter Questions
Problem 20.18QAP
Related questions
Question
Transcribed Image Text:18914186&UpenVellumHMAC=c662ec09ac8023bd1b971c039afd0082#10001
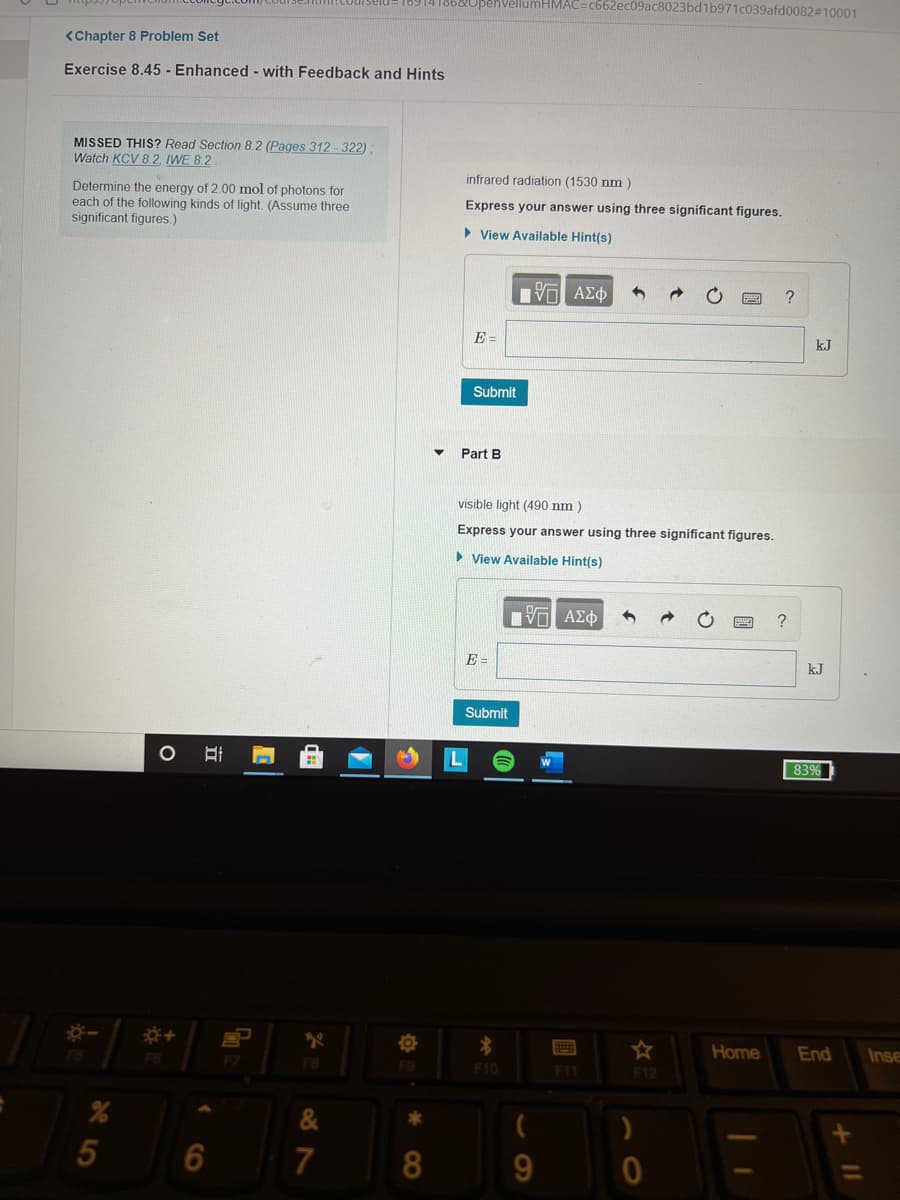
<Chapter 8 Problem Set
Exercise 8.45 - Enhanced - with Feedback and Hints
MISSED THIS? Read Section 8.2 (Pages 312-322);
Watch KCV 8.2, IWE 8.2
infrared radiation (1530 nm )
Determine the energy of 2.00 mol of photons for
each of the following kinds of light. (Assume three
significant figures.)
Express your answer using three significant figures.
> View Available Hint(s)
?
E =
kJ
Submit
Part B
visible light (490 nm )
Express your answer using three significant figures.
• View Available Hint(s)
E =
kJ
Submit
83%
Home
End
Inse
FB
F10
F11
F12
&
5
6
7
8.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
