Select the correct and balanced equation described by: An aqueous solution of lead(Il) sulfate is mixed with an aqueous solution of sodium chloride. + NaClag PbClac NazSO4 Pb(SOzia + 2NaClog PbCl, + 2NaSOd Pb(SOa)2lag) + 2NaClag PbCla + 2N2SO PbSO 4la) + 2NaClag) PbCl NazSO.)
Select the correct and balanced equation described by: An aqueous solution of lead(Il) sulfate is mixed with an aqueous solution of sodium chloride. + NaClag PbClac NazSO4 Pb(SOzia + 2NaClog PbCl, + 2NaSOd Pb(SOa)2lag) + 2NaClag PbCla + 2N2SO PbSO 4la) + 2NaClag) PbCl NazSO.)
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter12: Solutions
Section: Chapter Questions
Problem 12.31QP
Related questions
Question

Transcribed Image Text:Select the correct and balanced equation described by:
An aqueous solution of lead(II) sulfate is mixed with an aqueous solution of sodium chloride.
PBSO)
+ NaClag PbCl,+ NazSO4)
Pb(SO)2iag + 2NaClag PbCl2y + 2NaSOen
Pb(SOa)2lag) + 2NaClag PbCla, + 2N2SO
PbSO lag) + 2NaClag PbClay + NazSO.
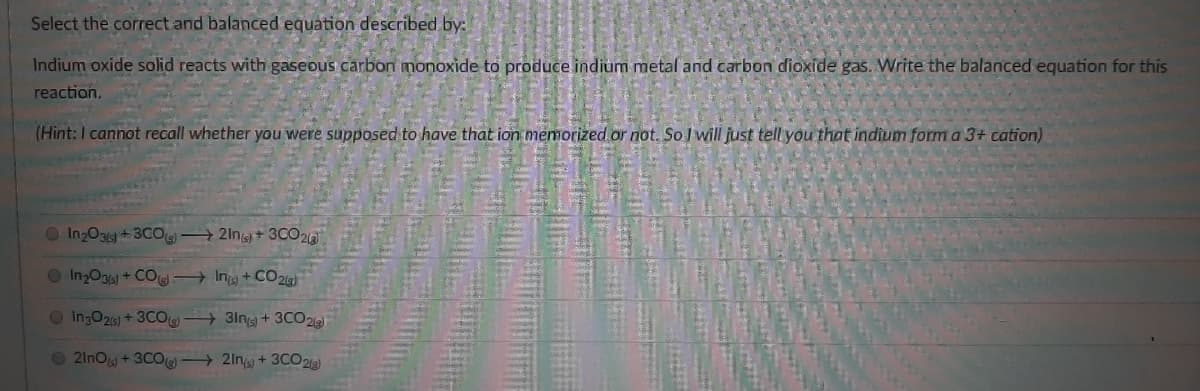
Transcribed Image Text:Select the correct and balanced equation described by:
Indium oxide solid reacts with gaseous carbon monoxide to produce indium metal and carbon dioxide gas. Write the balanced equation for this
reaction.
(Hint: I cannot recall whether you were supposed to have that ion memorized or not. So I will just tell you that indium form a 3+ cation)
O InzO3) +3COL
2In+ 3CO2a
O In2Og6) + COle + Ing + CO215)
OIngO26) + 3CO + 3lng + 3CO2
O21nO +3CO 2ing + 3CO21)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning