Silicon and carbon are both in Group 4 of the Periodic Table. They form the oxides silicon dioxide (SiO2) and carbon dioxide (CO2). The properties of these two oxides are shown in the table below: Carbon dioxide Sublimes at -78°C Sublimes at -78°C Silicon dioxide 1610°C Property Melting point Boiling point Electrical conductivity Give the structures and bonding of carbon dioxide and silicon dioxide. Explain your answers 2230°C рoor рoor
Silicon and carbon are both in Group 4 of the Periodic Table. They form the oxides silicon dioxide (SiO2) and carbon dioxide (CO2). The properties of these two oxides are shown in the table below: Carbon dioxide Sublimes at -78°C Sublimes at -78°C Silicon dioxide 1610°C Property Melting point Boiling point Electrical conductivity Give the structures and bonding of carbon dioxide and silicon dioxide. Explain your answers 2230°C рoor рoor
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 100E: A factory wants to produce 1.00 103 kg barium from the electrolysis of molten barium chloride. What...
Related questions
Question
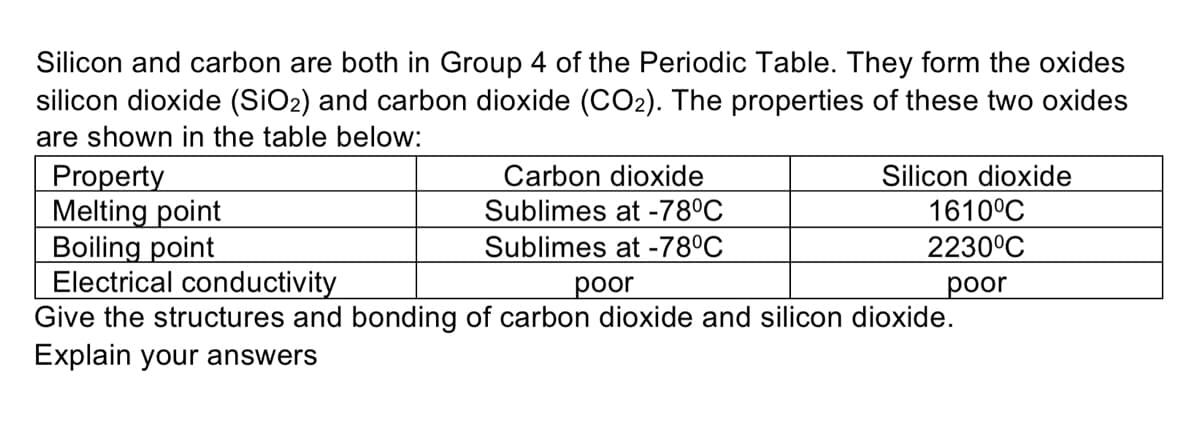
Transcribed Image Text:Silicon and carbon are both in Group 4 of the Periodic Table. They form the oxides
silicon dioxide (SiO2) and carbon dioxide (CO2). The properties of these two oxides
are shown in the table below:
Carbon dioxide
Sublimes at -78°C
Silicon dioxide
1610°C
Property
Melting point
Boiling point
Electrical conductivity
Give the structures and bonding of carbon dioxide and silicon dioxide.
Explain your answers
Sublimes at -78°C
2230°C
рoor
рoor

Transcribed Image Text:Na*
b)Use the diagram to explain why ...
i) sodium chloride crystals can be cubed shaped
ii) solid sodium chloride has a high melting point
iii) solid sodium chloride is an insulator but liquid sodium chloride will undergo electrolysis
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning