Sodium acetate dissolves readily in water according to the following equation. NaC2H3O2 (s) –→ NaC2H3O2 (aq) AH = -17.3 kJ Classify this process as either exothermic or endothermic and explain how you determined it. в I 0 / 10000 Word Limit 4 5 7 8 9 10 11 12 13 Nex
Sodium acetate dissolves readily in water according to the following equation. NaC2H3O2 (s) –→ NaC2H3O2 (aq) AH = -17.3 kJ Classify this process as either exothermic or endothermic and explain how you determined it. в I 0 / 10000 Word Limit 4 5 7 8 9 10 11 12 13 Nex
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.42E: A 244-g amount of coffee in an open plastic cup cools from 80.0C to 20.0C. Assuming no loss of mass...
Related questions
Question
100%
Transcribed Image Text:POSSIBLE POINTS: 10
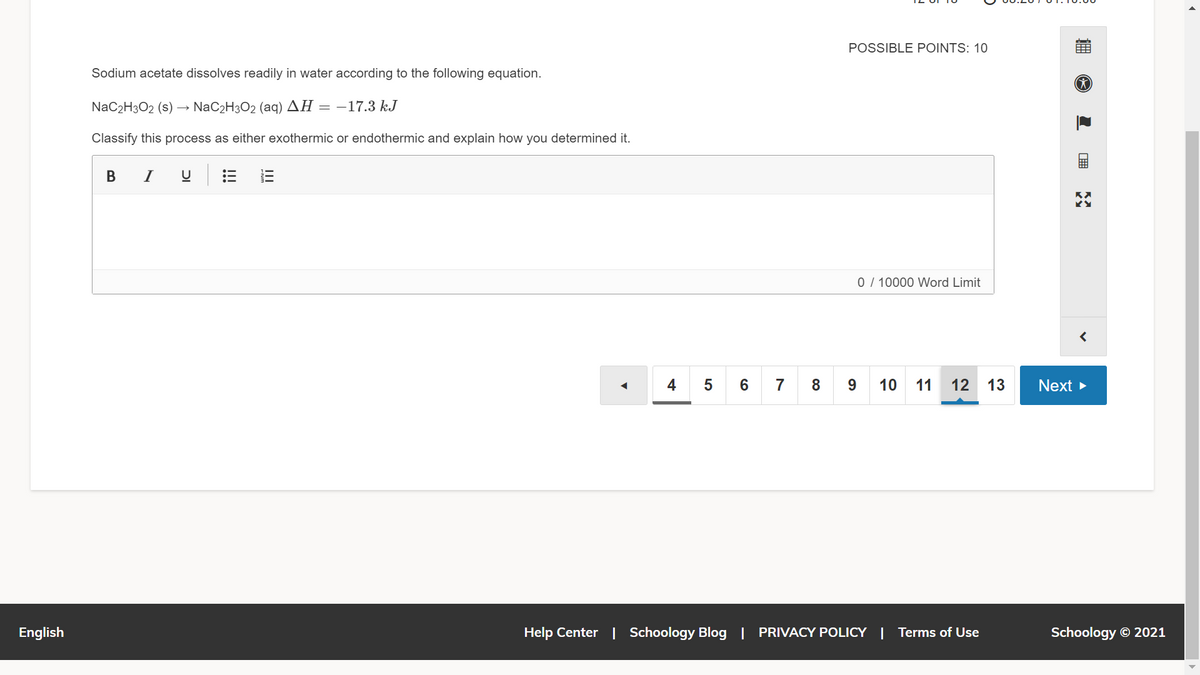
Sodium acetate dissolves readily in water according to the following equation.
NaC2H3O2 (s) → NaC2H3O2 (aq) AH = –17.3 kJ
Classify this process as either exothermic or endothermic and explain how you determined it.
B I
0 / 10000 Word Limit
4
7
8
9.
10
11
12
13
Next >
English
Help Center | Schoology Blog | PRIVACY POLICY | Terms of Use
Schoology © 2021
II
!!!
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning