Sodium hydroxide reacts with carbon dioxide as follows: 2NaOH(s)+CO2(g)→Na2CO3(s)+H2O(l) How many moles of Na2CO3Na2CO3 can be produced? Express your answer in moles to two decimal places.
Sodium hydroxide reacts with carbon dioxide as follows: 2NaOH(s)+CO2(g)→Na2CO3(s)+H2O(l) How many moles of Na2CO3Na2CO3 can be produced? Express your answer in moles to two decimal places.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter3: Molecules, Moles, And Chemical Equations
Section: Chapter Questions
Problem 3.15PAE: 3.15 Ethanol, C2H5OH is found in gasoline blends used in many parts of North America. Write a...
Related questions
Question
Sodium hydroxide reacts with carbon dioxide as follows:
2NaOH(s)+CO2(g)→Na2CO3(s)+H2O(l)
How many moles of Na2CO3Na2CO3 can be produced?
Express your answer in moles to two decimal places.
Transcribed Image Text:I Review I Constants I Periodic Table
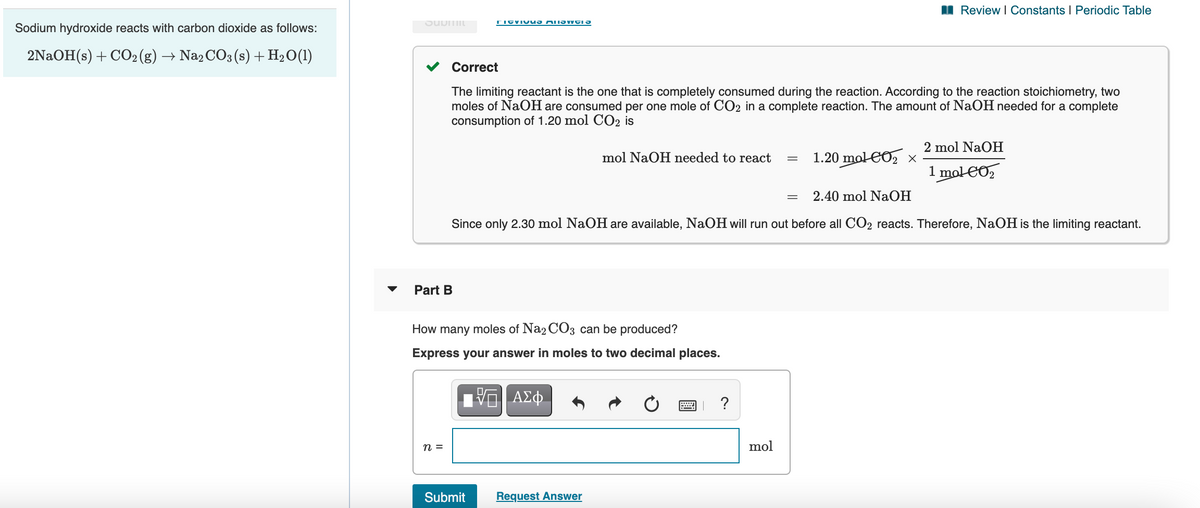
Sodium hydroxide reacts with carbon dioxide as follows:
2NAOH(s) + CO2 (g) → Na2 CO3 (s) + H2O(1)
Correct
The limiting reactant is the one that is completely consumed during the reaction. According to the reaction stoichiometry, two
moles of NaOH are consumed per one mole of CO2 in a complete reaction. The amount of NaOH needed for a complete
consumption of 1.20 mol CO2 is
2 mol NaOH
mol NaOH needed to react
1.20 mol CO, x
1 mol CO,
2.40 mol NaOH
Since only 2.30 mol NaOH are available, NaOH will run out before all CO2 reacts. Therefore, NaOH is the limiting reactant.
Part B
How many moles of Na2 CO3 can be produced?
Express your answer in moles to two decimal places.
?
n =
mol
Submit
Request Answer
Expert Solution
Step 1
Balanced reaction: A chemical reaction is said to be balanced when all number of atom at reactant and at product are equal. It is important to balance atom on both side because chemical reactions works on principle of law of conservation of mass, which states that during a chemical reaction atoms are not lost but they convert into stable molecules as desired product.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning