The drawing below shows a mixture of molecules: key carbon hydrogen nitrogen sulfur охудen chlorine Suppose the following chemical reaction can take place in this mixture: 2 H, (g)+O,(g) → 2 H, O(g) Of which reactant are there the most initial moles? Enter its chemical formula: Of which reactant are there the least initial moles? Enter its chemical formula: Which reactant is the limiting reactant? Enter its chemical formula:
The drawing below shows a mixture of molecules: key carbon hydrogen nitrogen sulfur охудen chlorine Suppose the following chemical reaction can take place in this mixture: 2 H, (g)+O,(g) → 2 H, O(g) Of which reactant are there the most initial moles? Enter its chemical formula: Of which reactant are there the least initial moles? Enter its chemical formula: Which reactant is the limiting reactant? Enter its chemical formula:
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.72PAE: 4.72 The picture shown depicts the species present at the start of a combustion reaction between...
Related questions
Question
100%
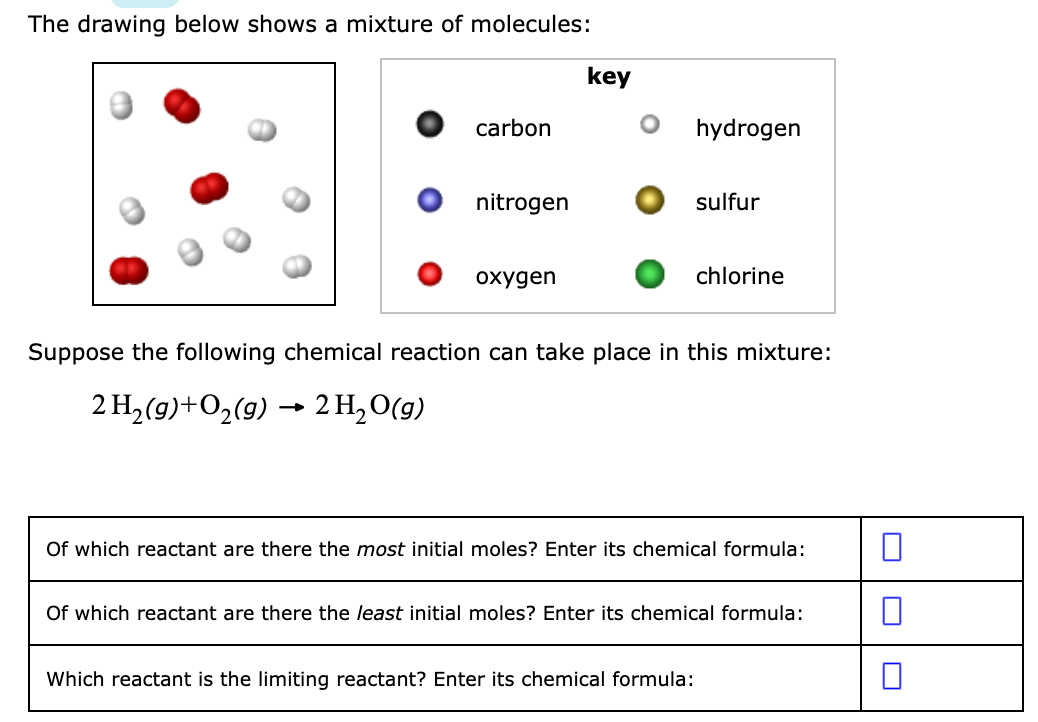
Transcribed Image Text:The drawing below shows a mixture of molecules:
key
carbon
hydrogen
nitrogen
sulfur
охудen
chlorine
Suppose the following chemical reaction can take place in this mixture:
2 H, (g)+O,(g) → 2 H, O(g)
Of which reactant are there the most initial moles? Enter its chemical formula:
Of which reactant are there the least initial moles? Enter its chemical formula:
Which reactant is the limiting reactant? Enter its chemical formula:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning