Specific Heats of Common Substances at 25 °C and 1 bar Specific Heats of Common Substances at 25 °C and 1 bar Substance Symbol (state) Specific Heat (J/g °C) Substance Symbol (state) Specific Heat (J/g °C) helium He(g) 5.193 air 1.007 water H2O(1) 4.184 охудen O2(g) 0.918 ethanol C2H6O(1) 2.376 aluminum Al(s) 0.897 ice H20(s) 2.093 (at -10 °C) carbon dioxide CO2(g) 0.853 water vapor H2O(g) 1.864 argon Ar(g) 0.522 nitrogen N2(g) 1.040 iron Fe(s) 0.449 copper Cu(s) 0.385 lead Pb(s) 0.130 gold Au(s) 0.129 silicon Si(s) 0.712
Specific Heats of Common Substances at 25 °C and 1 bar Specific Heats of Common Substances at 25 °C and 1 bar Substance Symbol (state) Specific Heat (J/g °C) Substance Symbol (state) Specific Heat (J/g °C) helium He(g) 5.193 air 1.007 water H2O(1) 4.184 охудen O2(g) 0.918 ethanol C2H6O(1) 2.376 aluminum Al(s) 0.897 ice H20(s) 2.093 (at -10 °C) carbon dioxide CO2(g) 0.853 water vapor H2O(g) 1.864 argon Ar(g) 0.522 nitrogen N2(g) 1.040 iron Fe(s) 0.449 copper Cu(s) 0.385 lead Pb(s) 0.130 gold Au(s) 0.129 silicon Si(s) 0.712
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter7: Chemical Energy
Section: Chapter Questions
Problem 109AE: A sample of nickel is heated to 99.8C and placed in a coffee-cup calorimeter containing 150.0 g...
Related questions
Question
A 360-g piece of rebar (a steel rod used for reinforcing concrete) is dropped into 425 mL of water at 24.0 °C. The final temperature of the water was measured as 42.7 °C. Calculate the initial temperature of the piece
of rebar. Assume the specific heat of steel is approximately the same as that for iron (Table 5.1), and that all heat transfer occurs between the rebar and the water (there is no heat exchange with the surroundings).
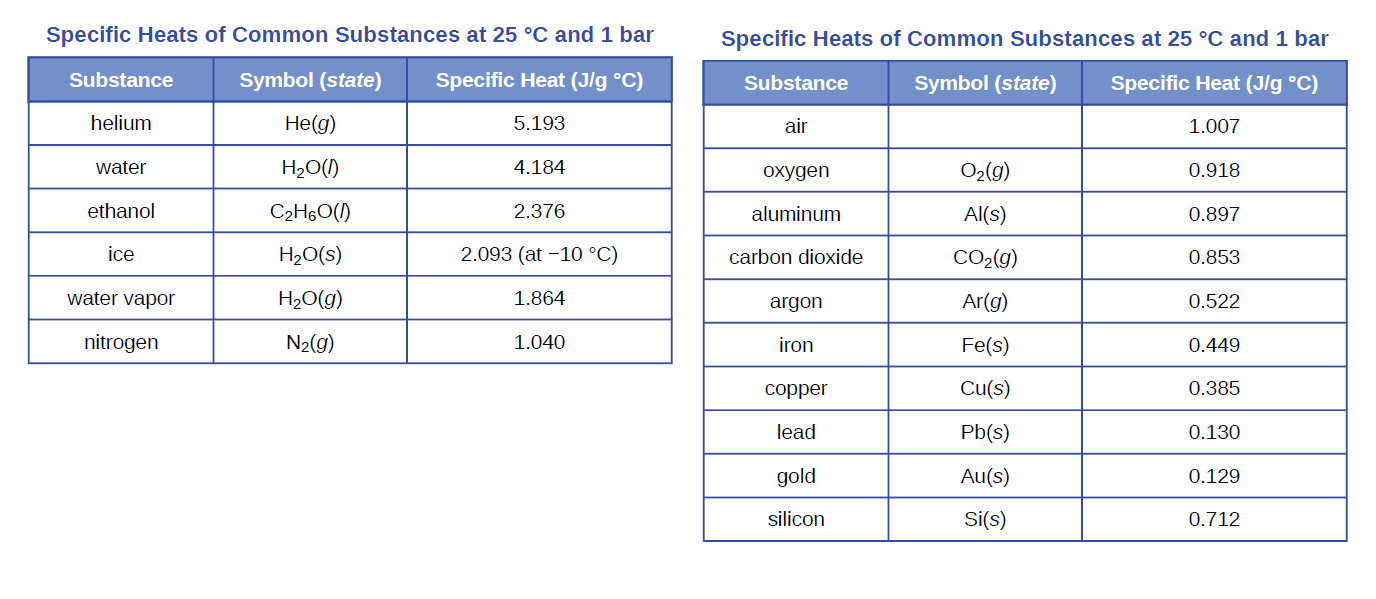
Transcribed Image Text:Specific Heats of Common Substances at 25 °C and 1 bar
Specific Heats of Common Substances at 25 °C and 1 bar
Substance
Symbol (state)
Specific Heat (J/g °C)
Substance
Symbol (state)
Specific Heat (J/g °C)
helium
He(g)
5.193
air
1.007
water
H2O(1)
4.184
охудen
O2(g)
0.918
ethanol
C2H6O(1)
2.376
aluminum
Al(s)
0.897
ice
H20(s)
2.093 (at -10 °C)
carbon dioxide
CO2(g)
0.853
water vapor
H2O(g)
1.864
argon
Ar(g)
0.522
nitrogen
N2(g)
1.040
iron
Fe(s)
0.449
copper
Cu(s)
0.385
lead
Pb(s)
0.130
gold
Au(s)
0.129
silicon
Si(s)
0.712
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning