standard enthalpy of the reaction, AH°, Calculate the rxn, for the thermite reaction: 2Al(s) + Fe2O3 (s)→2F€(s) + Al½O3(s) Elements in their standard state have an enthalpy of formation value of zero. The standard enthalpies of formation of Fe2O3 and Al203 are AĦ† of Fe2O3 (s) = -825.5 kJ/mol AĦt of Al2O3(s) = –1675 kJ/mol Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) НА ΔΗ, Value Units rxn =
standard enthalpy of the reaction, AH°, Calculate the rxn, for the thermite reaction: 2Al(s) + Fe2O3 (s)→2F€(s) + Al½O3(s) Elements in their standard state have an enthalpy of formation value of zero. The standard enthalpies of formation of Fe2O3 and Al203 are AĦ† of Fe2O3 (s) = -825.5 kJ/mol AĦt of Al2O3(s) = –1675 kJ/mol Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) НА ΔΗ, Value Units rxn =
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section5.8: Product- Or Reactant-favored Reactions And Thermodynamics
Problem 1.1ACP: The standard enthalpies of formation of KNO3(s) and K2S(s) are 494.6 kJ/mol and 376.6 kJ/mol,...
Related questions
Question
100%
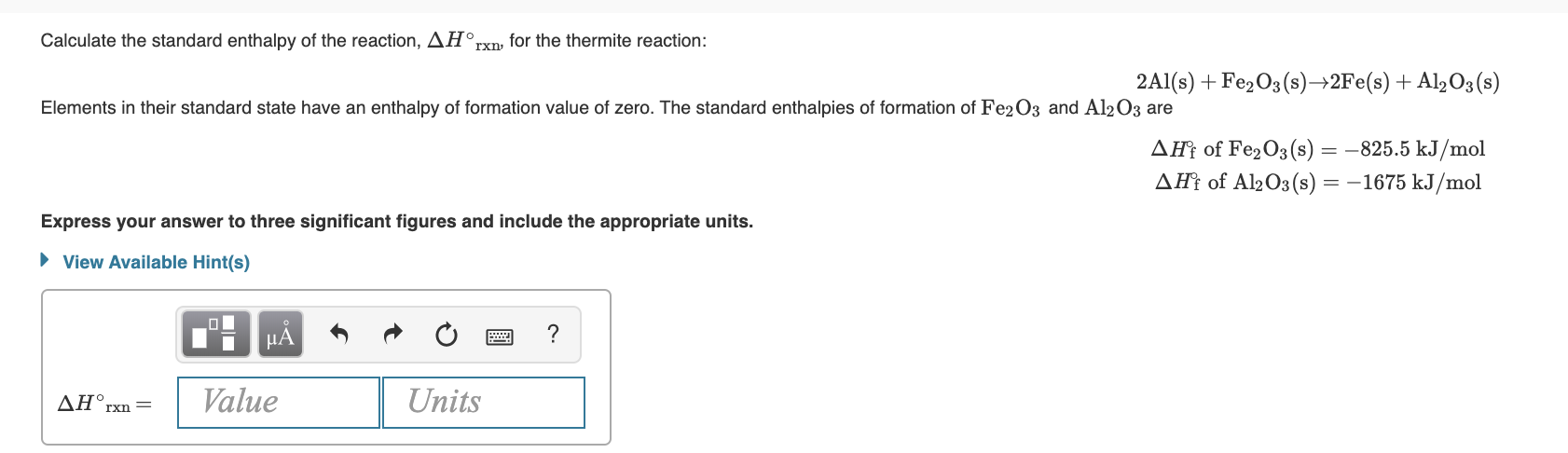
Transcribed Image Text:standard enthalpy of the reaction, AH°,
Calculate the
rxn, for the thermite reaction:
2Al(s) + Fe2O3 (s)→2F€(s) + Al½O3(s)
Elements in their standard state have an enthalpy of formation value of zero. The standard enthalpies of formation of Fe2O3 and Al203 are
AĦ† of Fe2O3 (s) = -825.5 kJ/mol
AĦt of Al2O3(s) = –1675 kJ/mol
Express your answer to three significant figures and include the appropriate units.
• View Available Hint(s)
НА
ΔΗ,
Value
Units
rxn =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co