STATEMENT OF THE PROBLEM Problem 2: Consider a 250.0 mL solution that contains 0.215 mole KNO3. What is the molar concentration of the 250.0 mL solution that contains 0.215 mole KNO3? STATEMENT OF THE PROBLEM Problem 3: A solution was prepared by diluting 25.0 mL of 1.42 M HCI to 500.0 mL. Write the mathematical equation for the dilution of a stock solution. Calculate the molarity of the resulting solution prepared by diluting 25.0 mL of 1.42 M HCI to 500.0 mL. "
STATEMENT OF THE PROBLEM Problem 2: Consider a 250.0 mL solution that contains 0.215 mole KNO3. What is the molar concentration of the 250.0 mL solution that contains 0.215 mole KNO3? STATEMENT OF THE PROBLEM Problem 3: A solution was prepared by diluting 25.0 mL of 1.42 M HCI to 500.0 mL. Write the mathematical equation for the dilution of a stock solution. Calculate the molarity of the resulting solution prepared by diluting 25.0 mL of 1.42 M HCI to 500.0 mL. "
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter7: Sollutions And Colloids
Section: Chapter Questions
Problem 7.1E: Many solutions are found in the home. Some are listed below, with the composition as printed on the...
Related questions
Question
solve problems 2 and 3 pls
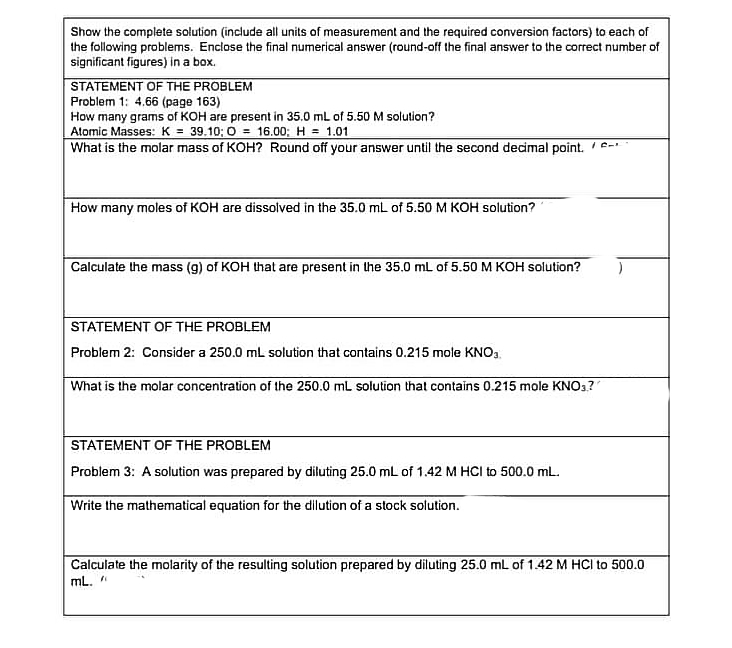
Transcribed Image Text:Show the complete solution (include all units of measurement and the required conversion factors) to each of
the following problems. Enclose the final numerical answer (round-off the final answer to the correct number of
significant figures) in a box.
STATEMENT OF THE PROBLEM
Problem 1: 4.66 (page 163)
How many grams of KOH are present in 35.0 mL of 5.50 M solution?
Alomic Masses: K = 39.10; 0 = 16.00; H = 1.01
What is the molar mass of KOH? Round off your answer until the second decimal point. /-
How many moles of KOH are dissolved in the 35.0 mL of 5.50 M KOH solution?
Calculate the mass (g) of KOH that are present in the 35.0 mL of 5.50 M KOH solution?
STATEMENT OF THE PROBLEM
Problem 2: Consider a 250.0 mL solution that contains 0.215 mole KNO3.
What is the molar concentration of the 250.0 mL solution that contains 0.215 mole KNO3?
STATEMENT OF THE PROBLEM
Problem 3: A solution was prepared by diluting 25.0 mL of 1.42 M HCI to 500.0 mL.
Write the mathematical equation for the dilution of a stock solution.
Calculate the molarity of the resulting solution prepared by diluting 25.0 mL of 1.42 M HCI to 500.0
mL. "
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

