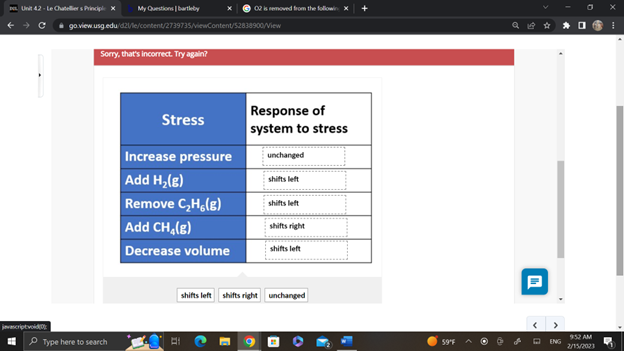
Stress Increase pressure Add H₂(g) Remove C₂H₂(g) Add CH (g) Decrease volume Response of system to stress unchanged shifts left shifts left shifts right shifts left shifts left shifts right unchanged
Stress Increase pressure Add H₂(g) Remove C₂H₂(g) Add CH (g) Decrease volume Response of system to stress unchanged shifts left shifts left shifts right shifts left shifts left shifts right unchanged
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter16: Solubility And Precipitation Equilibria
Section: Chapter Questions
Problem 37P
Related questions
Question
The following system is at equilibrium in a closed vessel:
2 CH4(g) ⇆ C2H6(g) + H2(g)
Various stresses are applied to the system as illustrated in the chart. Drag the appropriate label from the list below that indicates how the system responds to each stress. Each label can be used more than once.
Sorry, that's incorrect. Try again?
Transcribed Image Text:Unit 42-Le Chateliers Principle X
My Questions | bartleby
go.view.usg.edu/d21/le/content/2739735/viewContent/52838900/View
javascript:void(0
Sorry, that's incorrect. Try again?
Type here to search
Stress
Increase pressure
Add H₂(g)
Remove C₂H₂(g)
Add CH₂(g)
Decrease volume
02 is removed from the followin
Response of
system to stress
unchanged
shifts left
shifts left
shifts right
shifts left
shifts left shifts right unchanged
59°F
I
ENG
952 AM
2/15/2023
Expert Solution
Step 1

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning