Study the following reaction energy diagram: products energy reactants Then answer the following questions about the chemical reaction. O release O absorb Does this reaction release or absorb energy? neither How many transition states occur during this reaction? yes Could this be an elementary reaction? no said this reaction could not be entary, then how many steps are in echanism? u said this reaction could not be e ientary, then enter the number of the st in its mechanism which is rate- de ermining. For example, if the first step is the rate- there
Study the following reaction energy diagram: products energy reactants Then answer the following questions about the chemical reaction. O release O absorb Does this reaction release or absorb energy? neither How many transition states occur during this reaction? yes Could this be an elementary reaction? no said this reaction could not be entary, then how many steps are in echanism? u said this reaction could not be e ientary, then enter the number of the st in its mechanism which is rate- de ermining. For example, if the first step is the rate- there
Chapter8: Reaction Rates And Equilibrium
Section: Chapter Questions
Problem 8.20E
Related questions
Question
7
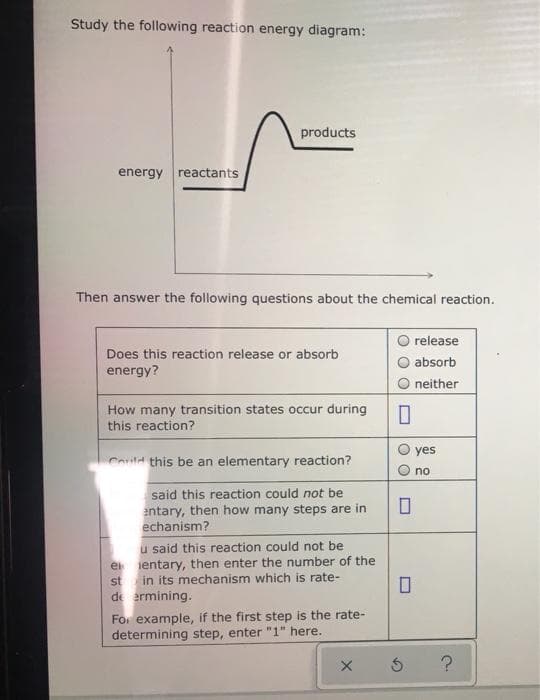
Transcribed Image Text:Study the following reaction energy diagram:
products
energy reactants
Then answer the following questions about the chemical reaction.
release
Does this reaction release or absorb
absorb
energy?
neither
How many transition states occur during
this reaction?
yes
Could this be an elementary reaction?
no
said this reaction could not be
entary, then how many steps are in
echanism?
u said this reaction could not be
e ientary, then enter the number of the
st in its mechanism which is rate-
de ermining.
For example, if the first step is the rate-
determining step, enter "1" here.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,