The graph shows three reaction pathways for the same biological reaction. One reaction pathway shows an uncatalyzed reaction, while two reaction pathways show catalyzed reactions: one catalyzed by enzyme X and one catalyzed by Y. For this particular biological reaction, enzyme X is a better catalyst than enzyme Y. Which statements apply to each reaction? Reactants Products Reaction pathway Drag each item to the appropriate bin. > View Available Hint(s) Reset Help catalyzed by enzyme X catalyzed by enzyme Y uncatalyzed reaction slowest reaction fastest reaction highest activation energy lowest activation energy Reaction A Reaction B Reaction C
The graph shows three reaction pathways for the same biological reaction. One reaction pathway shows an uncatalyzed reaction, while two reaction pathways show catalyzed reactions: one catalyzed by enzyme X and one catalyzed by Y. For this particular biological reaction, enzyme X is a better catalyst than enzyme Y. Which statements apply to each reaction? Reactants Products Reaction pathway Drag each item to the appropriate bin. > View Available Hint(s) Reset Help catalyzed by enzyme X catalyzed by enzyme Y uncatalyzed reaction slowest reaction fastest reaction highest activation energy lowest activation energy Reaction A Reaction B Reaction C
Chapter8: Reaction Rates And Equilibrium
Section: Chapter Questions
Problem 8.20E
Related questions
Question
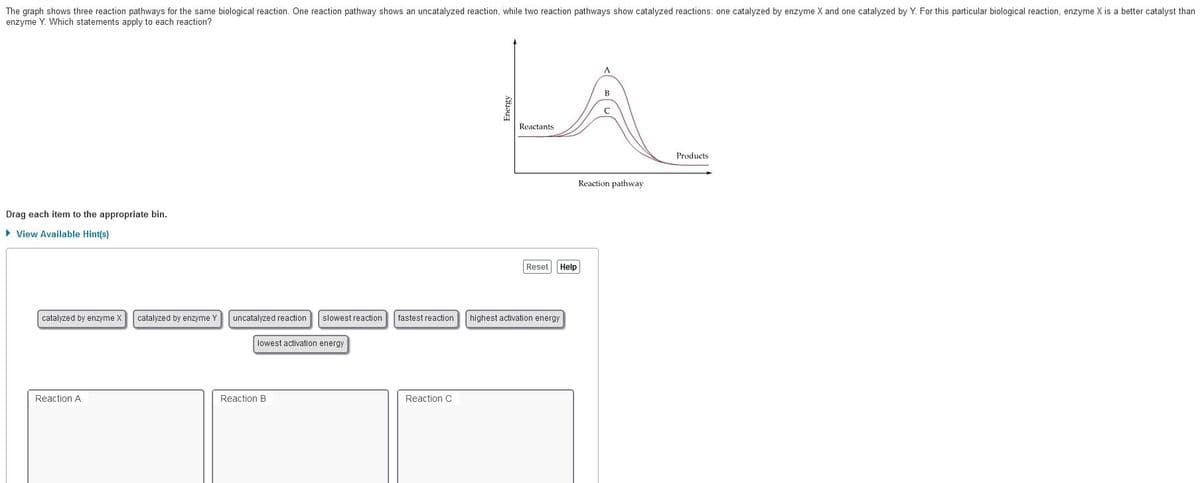
Transcribed Image Text:The graph shows three reaction pathways for the same biological reaction. One reaction pathway shows an uncatalyzed reaction, while two reaction pathways show catalyzed reactions: one catalyzed by enzyme X and one catalyzed by Y. For this particular biological reaction, enzyme X is a better catalyst than
enzyme Y. Which statements apply to each reaction?
A
Reactants
Products
Reaction pathway
Drag each item to the appropriate bin.
• View Available Hint(s)
Reset
Help
catalyzed by enzyme X
catalyzed by enzyme Y
uncatalyzed reaction
slowest reaction
fastest reaction
highest activation energy
lowest activation energy
Reaction A
Reaction B
Reaction C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning