Substance AH; (kJ/mol) Calculate the enthalpy change, AH°, for the "expansion" of methane: C(g) 718.4 CH4 (g) C(g) + 4H(g) CF4(g) Express your answer to one decimal place and include the appropriate units. -679.9 CH4(g) -74.8 > View Available Hint(s) H(g) 217.94 HF(g) -268.61 HA Keep in mind that the enthalpy of formation of an element in its standard state is zero. Value AH = Units
Substance AH; (kJ/mol) Calculate the enthalpy change, AH°, for the "expansion" of methane: C(g) 718.4 CH4 (g) C(g) + 4H(g) CF4(g) Express your answer to one decimal place and include the appropriate units. -679.9 CH4(g) -74.8 > View Available Hint(s) H(g) 217.94 HF(g) -268.61 HA Keep in mind that the enthalpy of formation of an element in its standard state is zero. Value AH = Units
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 65QAP: Natural gas companies in the United States use the therm as a unit of energy. One therm is 1105 BTU....
Related questions
Question
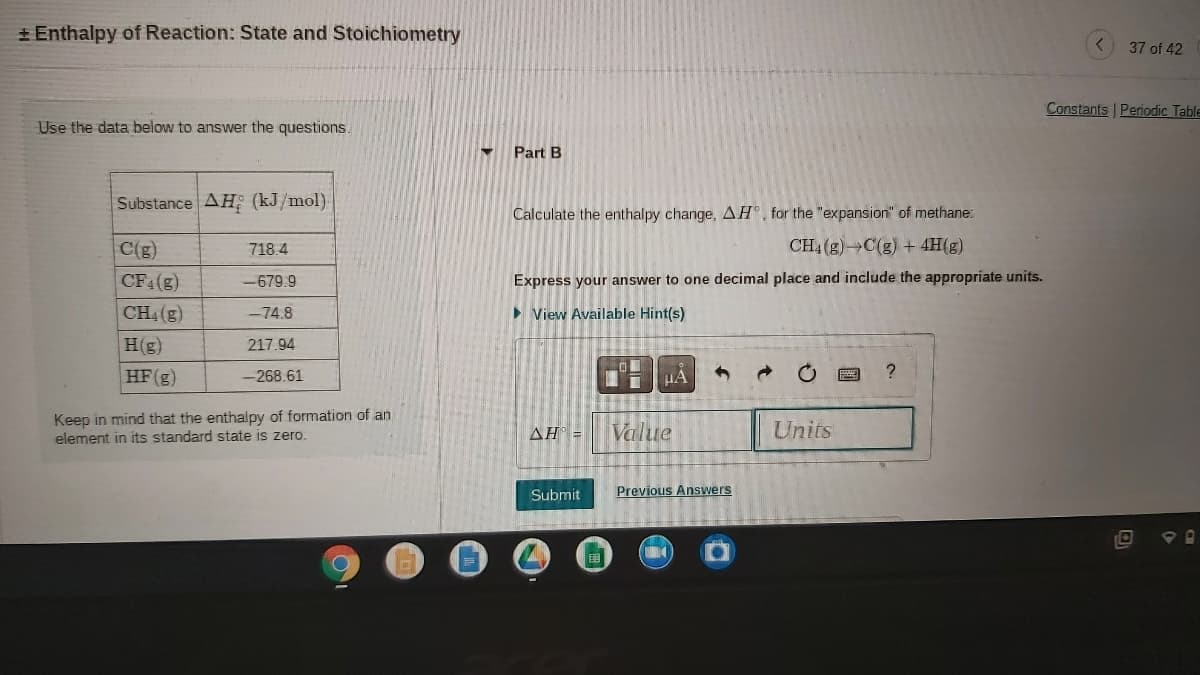
Transcribed Image Text:+ Enthalpy of Reaction: State and Stoichiometry
37 of 42
Constants Periodic Table
Use the data below to answer the questions.
Part B
Substance AH (kJ/mol)
Calculate the enthalpy change, AH®, for the "expansion" of methane:
C(g)
718.4
CH4 (g) C(g) + 4H(g)
CF4(g)
Express your answer to one decimal place and include the appropriate units.
-679.9
CH (g)
> View Available Hint(s)
-74.8
H(g)
217.94
HF(g)
-268.61
Keep in mind that the enthalpy of formation of an
element in its standard state is zero.
Value
Units
AH =
Submit
Previous Answers
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning