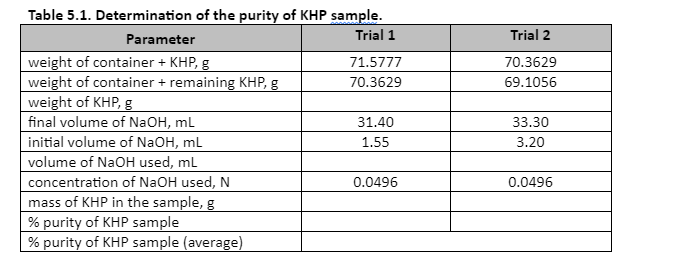
Table 5.1. Determination of the purity of KHP sample. Parameter Trial 1 Trial 2 weight of container + KHP, g weight of container + remaining KHP, g weight of KHP, g final volume of NaOH, mL 71.5777 70.3629 70.3629 69.1056 31.40 33.30 initial volume of NaOH, mL 1.55 3.20 volume of NaOH used, ml concentration of NaOH used, N mass of KHP in the sample, g % purity of KHP sample % purity of KHP sample (average) 0.0496 0.0496
Q: A pipet is used to transfer 3.00 mL of a 2.00 M stock solution in flask “S” to a 25.00-mL…
A: From the statement, it is given that Molarity of stock solution, M1= 2.00 M Let, Molarity of…
Q: Brand of Sample: Trial 1 Volume of sample 10.00 mL 25.63 ml Final reading - standard NAOH solution…
A: Vinegar is a dilute solution of acetic acid. It is used as salad dressing and preservative. The data…
Q: A 25.0 ml aliquot of a well-shaken and filtered sample of river water is pipetted into an…
A: Given data: Volume of sample = 25.0 mL, Mass of evaporating dish = 25.5 g, Mass of dried sample plus…
Q: A 15.0% by weight solution was prepared using 90.0g of KCl and the resulting density of the solution…
A: Given % by weight = 15 % Mass of solute = 90.0 g Density of solution = 1.101 g/ml
Q: The calibration curve was generated using known concentrations of five solutions of a newly…
A: The amount of a substance dissolved in a specified volume of the solvent produces a solution of a…
Q: Stock iron(II) solution (200Ug mL-1 Fe) ferrous ammonium sulfate hexahydrate mass= 0.1437g, transfer…
A: Given mass of ferrous ammonium sulfate hexahydrate = 0.1437g, Molar mass = 392.13 g/mol Total…
Q: To prepare 100ml of 0.2M from the stock solution (10M) we need to dilute 4ml of the stock solution…
A: To prepare a solution of given concentration from the stock solution, we need to use the molarity…
Q: Concentration iron(III) nitrate [Fe(NO3)31 (M) 0.35000 Color of iron(III) nitrate yellow…
A: Given, Concentration of iron (III) thiocyanate ion = 0.000250 M
Q: Before investigating the scene, the technician must dilute the luminol solution to a concentration…
A:
Q: 8.a) A solution has a gravity of 90° Tw. Calculate its specific gravity and its gravity in degree…
A: Of the three questions posted, only one question can be answered. The first question is more…
Q: An unknown sample containing a mixture of NaCl and Na2CO3 was analyzed to determine the %…
A: Here we have to determine the percentage of Na2CO3 in the mixture by given volumetric analysis data…
Q: How do I solve for the percentage of each substance in the mixture? What I know: Weight of…
A: % of any subastance in the mixture = weight of the substance in mixtureweight of mixture×100
Q: 21.929 Mass empty test tube and stopper Mass test tube, stopper and cyclohexanc Mass of cyclohexane…
A: Mass of cyclohexane(Solvent) = 6.39 g Mass of unknown in mixture 1 = 0.06 g' Mass of unknown in…
Q: In the standardization of HCl using pure anhydrous sodium carbonate as primary standard for methyl…
A: Here we have to determine the normality of HCl if in a titration 1ml of HCl is equivalent to 0.05 g…
Q: mass of dish 1631.5 g mass of dish and mix 1822 g mass of dish and agg. after extraction…
A: We have find out the volume of the solvent.
Q: B) Analysis of unknown aspirin tablet 1.Mass of aspirin tablet= 2.Concentration of ASA unknown from…
A: First, we have to draw the absorbance versus concentration(M) plot. From the linear regression…
Q: Solubility of an Unknown Solute 80 60 Solubility (g solute/ 100g H,0) 40 20 10 20 30 40 50 60 70 80…
A: Solubility curve is the graphical representation amount of solute dissolved in given amount of…
Q: Solution and explanation for Percentage Yield and Theoritical Yield for this data (Synthesis of…
A:
Q: 1) Mass of aspirin used (g) 0.088g 2) Absorbance for final unknown solution (measured) 3) Molarity…
A: Aspirin is a drug used for controlling pain, reducing fever etc. Aspirin tablet is not made up of…
Q: An aqueous solution of sodium acetate NaCH3COO, is made by dissolving 7.38 grams of sodium acetate…
A: Given data, Mass of sodium acetate=7.38 g Volume of sodium acetate solution = 200 ml
Q: What volume (in mL) of a 0.100 M HNO3 solution is required to completely react with 43.5 mL of a…
A:
Q: By pipet, 15.00ml of the stock solution of potassium permanganate (KMn04) that was prepared by…
A: Mass of KMnO4 = 13.0 gm, Molar mass of KMnO4 = 158.034 gm/mol Number of moles of KMnO4 =
Q: Commercial perchloric acid (100.46 g/mol) 71% (w/w) with specific gravity of 1.67 was diluted by…
A:
Q: Will Ag2 C03 (Ksp = 8.1 250.0ml of 0.0062 m 0.00014 m Na2 Co3 are
A: Solubility equilibrium of Ag2CO3 Ag2CO3(s) ----> 2Ag+(aq.) + CO32- Qsp = [Ag+]2[CO32-] =…
Q: 3. (a) If 10.0 mL of H;SO4 (sp. Gr. 1.50, containing 48.7% of combined SO3 by weight) is diluted to…
A: 3a) From the given data - 48.7 g of SO3 is present in 100 g of total solution. 10.0 mL…
Q: M = wt (g) 1000 M. wt (B v (mL) mol 0.250 M: wt (g) 1000 58.4 () 1 (mL) wt (g) 0.0146 B mL %3D…
A:
Q: What is the molarity of a concentrated solution of sulfuric acid with a specific gravity of 1.84 and…
A: Molarity is defined as the number of moles of solute per litre of solution. Molarity = Number of…
Q: Fill in the blanks: Computation: Round off answer (including partial calculation answers) to 4…
A: Here water is the solvent, and sucrose is the non-volatile solute. Given the vapor pressure of pure…
Q: #13 Mass percent of asprin in tablet
A: Since you have posted a question with multiple sub-parts, we will solve first three subparts for…
Q: An aqueous solution of sodium hydroxide contains 20.0% NaOH by mass. It is desired to produce an…
A:
Q: B'. Percent Water in a Hydrate [& # Waters per Formula Unit], Raw Data from Virtual Data Set Raw…
A: To determine the formula of hydrated salt we determine molar ratio of anhydrous salt and water.
Q: A pipet is used to transfer 5.00 mL of a 1.25 M stock solution in flask “S” to a 25.00-mL volumetric…
A: In the given problem, the molarity of the serially diluted solution can be calculated by using the…
Q: Balanced Chemical Equation MnO4- +5Fe2+ +8H+ --> Mn2+ +5Fe3+ +4H2O Details mass (g) sample average…
A: The balanced redox titration reaction is: MnO4-(aq) + 5Fe2+(aq) + 8H+(aq) →Mn2+(aq) + 5Fe3+(aq) +…
Q: diluting a 0.40M CuSO4 stock solution with water, calculate the concentration of each of the…
A: M1V1 = M2V2 M1 = concentration of CuSO4 =0.40 M V1 = volume of CuSO4 = 2.0 ml M2 = new…
Q: Masses of compounds used and observations NaCI CusO4-5H20 Bright blue crystaline solids…
A: Mass of the sodium Chloride used is eaqual to1. 2341
Q: 1. Volume of 0.10 M acetic acid needed for 25.0 mL of 0.020 M. mL 2. Molar mass of sodium acetate. g…
A: We’ll answer the first question since the exact one wasn’t specified. Please submit a new question…
Q: A pipet is used to transfer 5.00 mL of a 1.25 M stock solution in flask "S" to a 25.00-mL volumetric…
A: Molarity of a solution is the moles of solutes that is divided by the volume of the solution in…
Q: Density (g/mL) Reagent Salicylic acid Acetic anhydride Sulfuric acid Molar Mass (g/mole) Amount Mole…
A: The percentage yield of a product in a reaction is the percentage of that product obtained compared…
Q: In the standardization of HCl using pure anhydrous sodium carbonate as the primary standard for…
A: At the equivalence point, Amount of HCl = Amount of sodium carbonate Amount = Volume ×…
Q: In the distillation of the mixture of acetic acid (MW=60.05 g/mole; d=1.05g/ml) and water (MW= 18.00…
A: Density of acetic acid = 1.05 g/ml Density of water = 1.00 gm/ml Molarity of NaOH = 1.00 M Volume…
Q: Concentration Initial Final Volume Mass percent of acetic acid of Heinz volume Volume(mL) (mL) of in…
A:
Q: A pipet is used to transfer 5.00 mL of a 1.25 M stock solution in flask “S” to a 25.00-mL volumetric…
A: Molarity can be defined as the ratio of number of moles of solute present in a solution to the total…
Q: If 10.0 mL of H2SO4 (sp. Gr. 1.50, containing 48.7% of combined SO3 by weight) is diluted to 400 mL,…
A: A numerical problem based on concentration terms that is to be accomplished.
4 decimal places
Step by step
Solved in 2 steps with 2 images

- 1. Estimate the density of a 25-API gravity dead oil at 100 F.Downvoted for wrong solution. A river is carrying water containing 2000 mg/l Magnesium Chloride into a small lake. The lake has a naturally occurring Magnesium Chloride of 50 mg/l. If the river flow is 2500 Lmin and the lake flow rate is 1.5 m³.sec¹, what is the concentration of MgCl2 in the lake after the discharge point? Assume that the flows in the river and lake are completely mixed, that the salt is a conservative substance, and the system is at steady state.Use only the first decimal points (X.X) for atomic masses. R = 8.314 L*kPa/mole*K. In a reactor at 350.0 kPA and 95 °C, HCl adds to acetylene by the following reaction:2HCl(g) + C2H2(g) --> C2Cl2H4(g)39.1 g of HCl and 39.1 g of acetylene are placed into the reactor. What is the limiting reagent? What is the final volume of the system?
- Using the percent purity calculations, determine the percent yield of synthesis of aspirin. Part I Synthesis of Aspirin Mass of salicylic acid used (g) 2.029g Volume of acetic anhydride used (mL) 5ml Mass of acetic anhydride used (vol. × 1.08 g/mL) 5.4g Mass of aspirin synthesized (g) 3.256g Part II Melting Temperature Data Melting temperature (°C) 133°C Part III Salicylic Acid Standard Stock Solution Initial mass of salicylic acid (g) 0.210g Moles of salicylic acid (mol) 0.0147 mol Initial molarity of salicylic acid (M) 0.724 M Part III Beer’s Law Data for Salicylic Acid Standard Solutions Trial Concentration (M) Absorbance Water (mL) 1 10 0.301 0 2 7.5 0.219 2.5 3 5.0 0.163 5.0 4 2.5 0.074 7.5 Best-fit line equation for the salicylic acid standards Test of the Purity of the Synthesized Aspirin Initial mass of aliquot of product (g)…There's 1 drink (and you are asked to determine the glucose concentration in the drink in the units of g/100mL. (Why these units? Well, once you have the concentrations in g/100mL you will be able to compare your values with the nutritional values given on the drink bottles’ labels). The sample of the drink was diluted 1/100 (i.e. by a factor of 100). This was an essential step in the method because, without it, the machine used to analyse the glucose concentration (spectrophotometer) would have given an error as the concentration would have been too high for accurate detection. What this means for you is that the dilution factor will need to be taken into consideration in your calculations (remember the aim is to calculate the concentration in the original drink and not in the diluted drink). You measured the concentration of their diluted drink using the spectrophotometer and their results were provided to them in the units mM (millimolar). Glucose Concentration in mM of drink =…There's 1 drink (and you are asked to determine the glucose concentration in the drink in the units of g/100mL. (Why these units? Well, once you have the concentrations in g/100mL you will be able to compare your values with the nutritional values given on the drink bottles’ labels). The sample of the drink was diluted 1/100 (i.e. by a factor of 100). This was an essential step in the method because, without it, the machine used to analyse the glucose concentration (spectrophotometer) would have given an error as the concentration would have been too high for accurate detection. What this means for you is that the dilution factor will need to be taken into consideration in your calculations (remember the aim is to calculate the concentration in the original drink and not in the diluted drink). You measured the concentration of their diluted drink using the spectrophotometer and their results were provided to them in the units mM (millimolar). Glucose Concentration in mM of drink =…
- 150 kmol of an aqueous phosphoric acid solution contains S mol percent H3P04. The solution is concentrated by adding pure phosphoric acid at a rate of 20L per minute. Write a differential mole balance on phosphoric acid and provide an initial condition. Solve the balance to abtain an expression vdor np(t) . Use the result to derive an expression far xp(t), the mole fraction of phosphoric acid in the solution. How long will it take to concentrate the solution to IS percent H3P04 ?Penicillin is hydrolyzed and thereby rendered inactive by beta-lactamase, an enzyme present in some penicillin-resistant bacteria. The mass of this enzyme is 30.0 kDa. The amount of penicillin hydrolyzed in 1 minute in a 1 mL solution containing 3 x 10-3 g of the enzyme was measured as a function of the concentration of penicillin. Calculate Km, Vmax , and kcat from the following data: (Clearly show where 1/Vmax and -1/Km are; clearly explain how to obtain your kcat value). [S] (mM) vo (mM s-1) 0.14 0.138 0.28 0.234 0.55 0.333 0.77 0.388 1.52 0.491A solution containing 100 lbm KNO 3/100 lbm H 2O at 80°C is fed to a cooling crystallizer operated at 25°C. Slurry from the crystallizer (KNO3 crystals suspended in saturated solution) is fed to a filter, where the crystals are separated from the solution. Use the solubility data in Figure 6.5-1 to determine the production rate of crystals (lbm/lbm feed) and the solid-to-liquid mass ratio (lbm crystals/lbm liquid) in the slurry leaving the crystallizer.
- A mixture of ethanol (ethyl alcohol) and water contains 40.0% water by mass.(a) Assuming volume additivity of the components, estimate the specific gravity of themixture at 20°C. What volume (in liters) of this mixture is required to provide 150 mol ofethanol?(b) Repeat Part (a) with the additional information that the specific gravity of the mixture at20°C is 0.89045 (making it unnecessary to assume volume additivity). What percentage errorresults from the volume-additivity assumption?What is the Molecular weight of a certain volatile liquid which is placed in 225 g flask which has total capacity volume of 252.17 mL. After the liquid was heated to 96.7 oC the gass volatilized and the weight of the flask measure 227.33g. What is the could be the MW of the volatile sample?Sum of coefficients C7H8 + O2 --> CO2 + H2O after balancing

