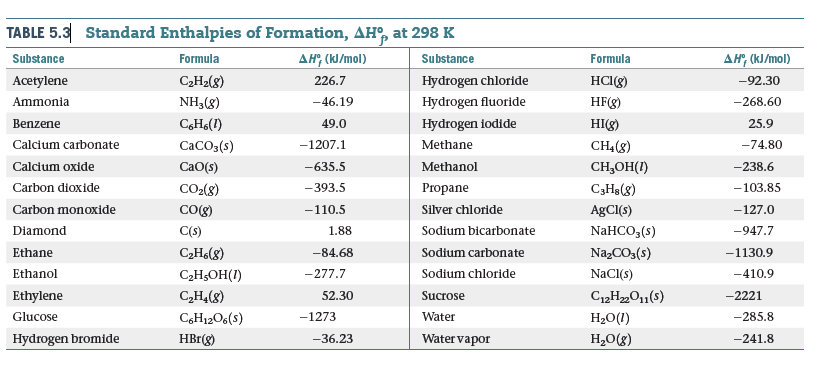
TABLE 5.3 Standard Enthalpies of Formation, AH, at 298 K дн, (КJ/mol) Substance Formula дн (К/mol) Substance Formula CH2(8) Acetylene HCI(g) 226.7 Hydrogen chloride -92.30 Ammonia NH3(8) Hydrogen fluoride -46.19 HF(g) -268.60 CHo(1) Hydrogen iodide HI(g) 25.9 Benzene 49.0 CaCOs(s) -1207.1 Calcium carbonate Methane CH(8) -74.80 CaO(s) Methanol CH,ОН() Calcium oxide -635.5 -238.6 CO2(8) Carbon dioxide -103.85 -393.5 Propane C3Hs(8) Carbon monoxide CO(g) -110.5 Silver chloride AgCI(s) -127.0 Sodium bicarbonate Diamond C(s) 1.88 NaHCO,(s) -947.7 C2H6(8) NazCO3(s) Ethane -84.68 Sodium carbonate -1130.9 NACI(s) Ethanol CэHsОН() -277.7 Sodium chloride -410.9 Ethylene C,H4(8) C12H„O1(s) 52.30 Sucrose -2221 Water Glucose CH12O6(s) -1273 H20(1) -285.8 Hydrogen bromide Water vapor H,O(g) HBr(g) -36.23 -241.8
Thermochemistry
Thermochemistry can be considered as a branch of thermodynamics that deals with the connections between warmth, work, and various types of energy, formed because of different synthetic and actual cycles. Thermochemistry describes the energy changes that occur as a result of reactions or chemical changes in a substance.
Exergonic Reaction
The term exergonic is derived from the Greek word in which ‘ergon’ means work and exergonic means ‘work outside’. Exergonic reactions releases work energy. Exergonic reactions are different from exothermic reactions, the one that releases only heat energy during the course of the reaction. So, exothermic reaction is one type of exergonic reaction. Exergonic reaction releases work energy in different forms like heat, light or sound. For example, a glow stick releases light making that an exergonic reaction and not an exothermic reaction since no heat is released. Even endothermic reactions at very high temperature are exergonic.
Diethyl ether, C4H10O(l), a flammable compound that was
once used as a surgical anesthetic, has the structure
H3C-CH2- O- CH2-0 CH3
The complete combustion of 1 mol of C4H10O1l2 to CO21g2
and H2O(l) yields ΔH° = -2723.7 kJ. (a) Write a balanced
equation for the combustion of 1 mol of C4H10O(l). (b) By
using the information in this problem and data in Table 5.3,
calculate ΔHf° for diethyl ether.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images









