TABLE 5.4 Average Bond Enthalpies (kJ/mol) 391 413 N-H 0-H 463 155 348 163 0-0 146 614 253 C=C 201 495 Cl-F C-N 190 242 293 N-F 272 0-F Cl-CI N-CI 358 200 0-CI 203 799 N-Br 243 0-I 234 Br-F 237 C-F 485 Br-Cl 218 С—СІ 328 Н-н 436 Br –Br 193 С -Вг 276 H-F 567 431 208 C-I 240 Н—СІ I-CI 1— Вг 175 Н-Br 366 Н—І 299 I-I 151 APPENDIX C Thermodynamic Quantitles for Selected Substances at 298.15 K (25 "C) 1091 дн; (kj/mol) AG; S* S° дн AG; (kj/mol) -245.6 (kj/mol) (i/mol-K) Substance Substance (i/mol-K) (kj/mol) Vanadium SOCI (1) H,S(8) H2SO4(aq) H,SO,(1) 205.6 20.1 182.2 -20.17 -33.01 514.2 453.1 V(g) V(s) -909.3 -744.5 28.9 156.1 -689.9 -814.0 Zinc Titanium 130.7 95.2 160.9 Zn(g) Ti(g) Ti(s) 468 180.3 422 41.63 Zn(s) ZnCl,(s) ZnO(s) -369.4 -415.1 111.5 30.76 -763.2 -726.8 354.9 TICL4(8) TICI4(1) TIO2(s) -318.2 -348.0 43.9 -804.2 -728.1 221.9 -889.4 50.29 -944.7
TABLE 5.4 Average Bond Enthalpies (kJ/mol) 391 413 N-H 0-H 463 155 348 163 0-0 146 614 253 C=C 201 495 Cl-F C-N 190 242 293 N-F 272 0-F Cl-CI N-CI 358 200 0-CI 203 799 N-Br 243 0-I 234 Br-F 237 C-F 485 Br-Cl 218 С—СІ 328 Н-н 436 Br –Br 193 С -Вг 276 H-F 567 431 208 C-I 240 Н—СІ I-CI 1— Вг 175 Н-Br 366 Н—І 299 I-I 151 APPENDIX C Thermodynamic Quantitles for Selected Substances at 298.15 K (25 "C) 1091 дн; (kj/mol) AG; S* S° дн AG; (kj/mol) -245.6 (kj/mol) (i/mol-K) Substance Substance (i/mol-K) (kj/mol) Vanadium SOCI (1) H,S(8) H2SO4(aq) H,SO,(1) 205.6 20.1 182.2 -20.17 -33.01 514.2 453.1 V(g) V(s) -909.3 -744.5 28.9 156.1 -689.9 -814.0 Zinc Titanium 130.7 95.2 160.9 Zn(g) Ti(g) Ti(s) 468 180.3 422 41.63 Zn(s) ZnCl,(s) ZnO(s) -369.4 -415.1 111.5 30.76 -763.2 -726.8 354.9 TICL4(8) TICI4(1) TIO2(s) -318.2 -348.0 43.9 -804.2 -728.1 221.9 -889.4 50.29 -944.7
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.37QP
Related questions
Question
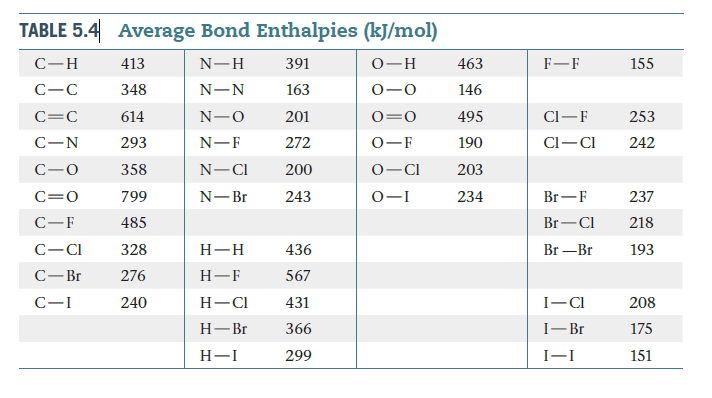
Consider the reaction H2(g) + I2(s)---->2 HI(g). (a) Use the
bond enthalpies in Table 5.4 to estimate ΔH for this reaction,
ignoring the fact that iodine is in the solid state. (b) Without
doing a calculation, predict whether your estimate in part (a)
is more negative or less negative than the true reaction enthalpy.
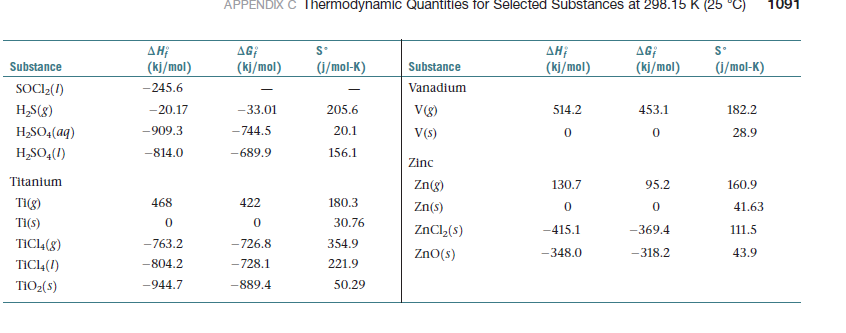
(c) Use the enthalpies of formation in Appendix C to
determine the true reaction enthalpy.
Transcribed Image Text:TABLE 5.4 Average Bond Enthalpies (kJ/mol)
391
413
N-H
0-H
463
155
348
163
0-0
146
614
253
C=C
201
495
Cl-F
C-N
190
242
293
N-F
272
0-F
Cl-CI
N-CI
358
200
0-CI
203
799
N-Br
243
0-I
234
Br-F
237
C-F
485
Br-Cl
218
С—СІ
328
Н-н
436
Br –Br
193
С -Вг
276
H-F
567
431
208
C-I
240
Н—СІ
I-CI
1— Вг
175
Н-Br
366
Н—І
299
I-I
151
Transcribed Image Text:APPENDIX C Thermodynamic Quantitles for Selected Substances at 298.15 K (25 "C)
1091
дн;
(kj/mol)
AG;
S*
S°
дн
AG;
(kj/mol)
-245.6
(kj/mol)
(i/mol-K)
Substance
Substance
(i/mol-K)
(kj/mol)
Vanadium
SOCI (1)
H,S(8)
H2SO4(aq)
H,SO,(1)
205.6
20.1
182.2
-20.17
-33.01
514.2
453.1
V(g)
V(s)
-909.3
-744.5
28.9
156.1
-689.9
-814.0
Zinc
Titanium
130.7
95.2
160.9
Zn(g)
Ti(g)
Ti(s)
468
180.3
422
41.63
Zn(s)
ZnCl,(s)
ZnO(s)
-369.4
-415.1
111.5
30.76
-763.2
-726.8
354.9
TICL4(8)
TICI4(1)
TIO2(s)
-318.2
-348.0
43.9
-804.2
-728.1
221.9
-889.4
50.29
-944.7
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning