1. Use the bond enthalpies to calculate the enthalpy change for this reaction. Is the reaction endothermic or exothermic? Show all of your work. | CHỌN + NH, → CH₂ + N₂ + H₂ н H-N-H H-C-H N=N H-C= N-H H H H Bond Breaking Bond Making H-H TABLE 8.6 Average Bond Dissociation Energies and Bond Lengths for Some Common Covalent Bonds Energy Length Energy Length Bond (kJ/mol) (pm) Bond (kJ/mol) (pm) Bond Energy Length (kJ/mol) (pm) H-H 432 75 N-H 391 101 F-F 154 142 H-F 565 92 N-F 272 136 F-C 253 165 H-a 427 127 N-CI 200 175 F-Br 237 176 H-Br 363 141 N-Br 243 189 F-I 273 191 H-I 295 161 N-N 160 145 Cl-Cl 239 199 C-H 413 109 N=N 418 125 Cl-Br 218 214 C-F 485 135 N=N 941 110 CH-I 208 232 C-Cl 339 177 0-H 467 96 Br-Br 193 228 276 194 0-F 190 142 Br-I 175 247 240 214 0-Cl 203 172 H-I 149 267 347 154 0-1 234 206 S-H 363 134 614 134 0-0 146 148 S-F 327 156 839 120 495 121 S-Cl 253 207 305 143 C-O 358 143 S-Br 218 216 615 138 C=0 745* 120 S-S 226 205 C=N 891 116 C=0 1072 113 C=O bond energy in CO₂ is 799 kJ/mol.
1. Use the bond enthalpies to calculate the enthalpy change for this reaction. Is the reaction endothermic or exothermic? Show all of your work. | CHỌN + NH, → CH₂ + N₂ + H₂ н H-N-H H-C-H N=N H-C= N-H H H H Bond Breaking Bond Making H-H TABLE 8.6 Average Bond Dissociation Energies and Bond Lengths for Some Common Covalent Bonds Energy Length Energy Length Bond (kJ/mol) (pm) Bond (kJ/mol) (pm) Bond Energy Length (kJ/mol) (pm) H-H 432 75 N-H 391 101 F-F 154 142 H-F 565 92 N-F 272 136 F-C 253 165 H-a 427 127 N-CI 200 175 F-Br 237 176 H-Br 363 141 N-Br 243 189 F-I 273 191 H-I 295 161 N-N 160 145 Cl-Cl 239 199 C-H 413 109 N=N 418 125 Cl-Br 218 214 C-F 485 135 N=N 941 110 CH-I 208 232 C-Cl 339 177 0-H 467 96 Br-Br 193 228 276 194 0-F 190 142 Br-I 175 247 240 214 0-Cl 203 172 H-I 149 267 347 154 0-1 234 206 S-H 363 134 614 134 0-0 146 148 S-F 327 156 839 120 495 121 S-Cl 253 207 305 143 C-O 358 143 S-Br 218 216 615 138 C=0 745* 120 S-S 226 205 C=N 891 116 C=0 1072 113 C=O bond energy in CO₂ is 799 kJ/mol.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter8: Advanced Theories Of Covalent Bonding
Section: Chapter Questions
Problem 2E: Draw a curve that describes the energy of a system with H and Cl atoms at varying distances. Then,...
Related questions
Question
can this please be written on a paper
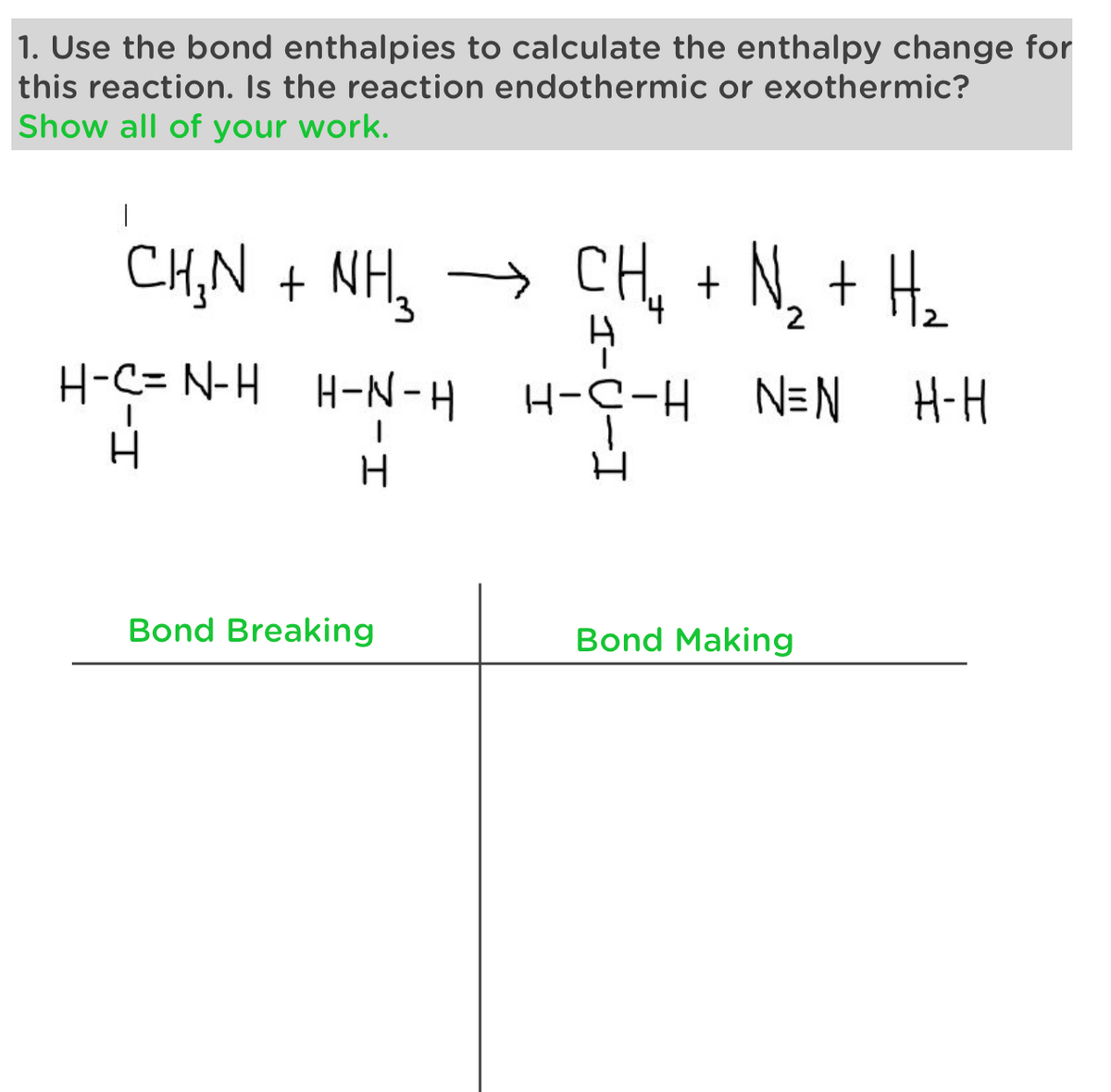
Transcribed Image Text:1. Use the bond enthalpies to calculate the enthalpy change for
this reaction. Is the reaction endothermic or exothermic?
Show all of your work.
|
CHỌN + NH,
→ CH₂ + N₂ + H₂
н
H-N-H H-C-H N=N
H-C= N-H
H
H
H
Bond Breaking
Bond Making
H-H
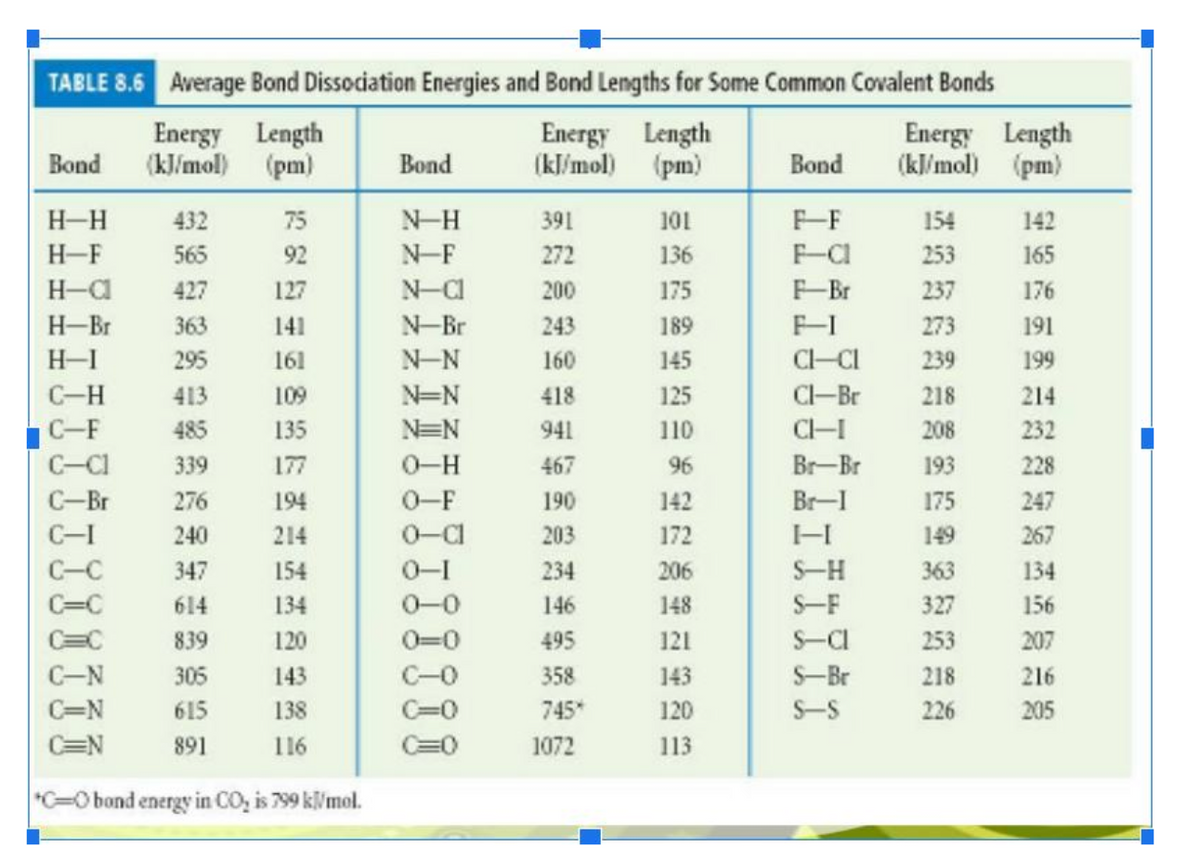
Transcribed Image Text:TABLE 8.6 Average Bond Dissociation Energies and Bond Lengths for Some Common Covalent Bonds
Energy Length
Energy Length
Bond
(kJ/mol) (pm)
Bond
(kJ/mol) (pm)
Bond
Energy Length
(kJ/mol) (pm)
H-H 432
75
N-H
391
101
F-F
154
142
H-F
565
92
N-F
272
136
F-C
253
165
H-a
427
127
N-CI
200
175
F-Br
237
176
H-Br
363
141
N-Br
243
189
F-I
273
191
H-I
295
161
N-N
160
145
Cl-Cl
239
199
C-H
413
109
N=N
418
125
Cl-Br
218
214
C-F
485
135
N=N
941
110
CH-I
208
232
C-Cl
339
177
0-H
467
96
Br-Br
193
228
276
194
0-F
190
142
Br-I
175
247
240
214
0-Cl
203
172
H-I
149
267
347
154
0-1
234
206
S-H
363
134
614
134
0-0
146
148
S-F
327
156
839
120
495
121
S-Cl
253
207
305
143
C-O
358
143
S-Br
218
216
615
138
C=0
745*
120
S-S
226
205
C=N
891
116
C=0
1072
113
C=O bond energy in CO₂ is 799 kJ/mol.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 1 steps with 1 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning