Talc, Mg3Si4O10(OH)2, reacts with forsterite (Mg,SiO4) to form enstatite (MgSiO3) and water vapor. (a) Write a balanced chemical equation for this reaction. (b) If the water pressure is equal to the total pressure, will formation of products be favored or disfavored with increasing total pressure? (c) The entropy change for this reaction is positive. Will the slope of the coexistence curve (pressure plotted against temperature) be positive or negative?
Talc, Mg3Si4O10(OH)2, reacts with forsterite (Mg,SiO4) to form enstatite (MgSiO3) and water vapor. (a) Write a balanced chemical equation for this reaction. (b) If the water pressure is equal to the total pressure, will formation of products be favored or disfavored with increasing total pressure? (c) The entropy change for this reaction is positive. Will the slope of the coexistence curve (pressure plotted against temperature) be positive or negative?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter13: Spontaneous Processes And Thermodynamic Equilibrium
Section: Chapter Questions
Problem 22P: Use data from Appendix D to calculate the standardentropy change at 25°C for the reaction...
Related questions
Question
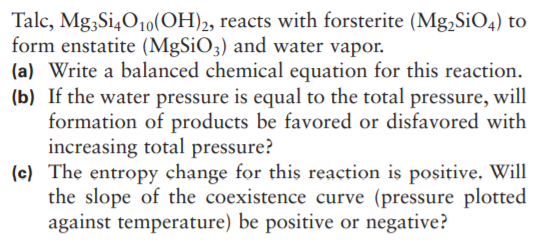
Transcribed Image Text:Talc, Mg3Si4O10(OH)2, reacts with forsterite (Mg,SiO4) to
form enstatite (MgSiO3) and water vapor.
(a) Write a balanced chemical equation for this reaction.
(b) If the water pressure is equal to the total pressure, will
formation of products be favored or disfavored with
increasing total pressure?
(c) The entropy change for this reaction is positive. Will
the slope of the coexistence curve (pressure plotted
against temperature) be positive or negative?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning