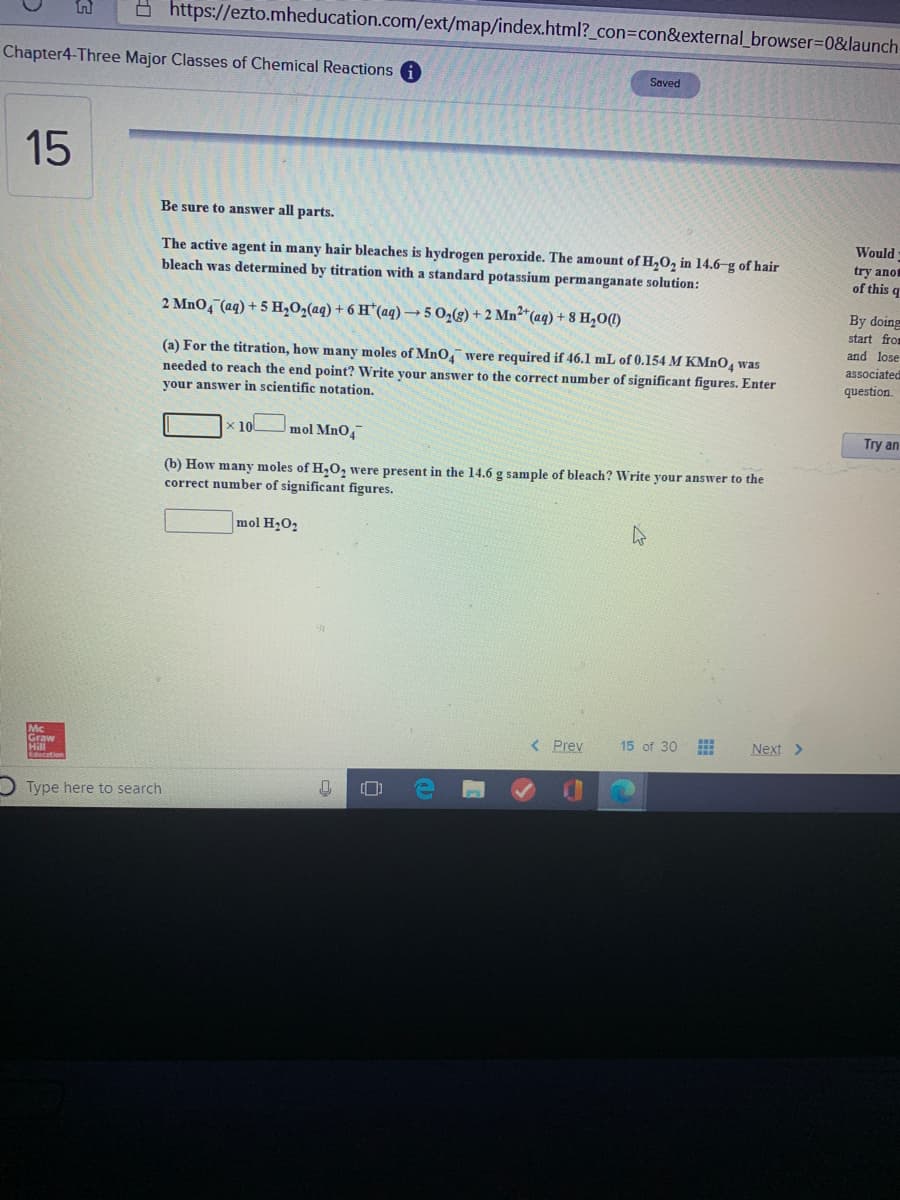
The active agent in many hair bleaches is hydrogen peroxide. The amount of H,O, in 14.6-g of hair bleach was determined by titration with a standard potassium permanganate solution: 2 MnO, (aq) + 5 H,O2(aq) + 6 H*(aq)→50½(g) + 2 Mn²*(aq) + 8 H,0(1) (a) For the titration, how many moles of MnO, were required if 46.1 mL of 0.154 M KMNO, was needed to reach the end point? Write your answer to the correct number of significant figures. Enter your answer in scientific notation. x 10 mol MnO, (b) How many moles of H,0, were present in the 14.6 g sample of bleach? Write your answer to the correct number of significant figures. mol H;O2
The active agent in many hair bleaches is hydrogen peroxide. The amount of H,O, in 14.6-g of hair bleach was determined by titration with a standard potassium permanganate solution: 2 MnO, (aq) + 5 H,O2(aq) + 6 H*(aq)→50½(g) + 2 Mn²*(aq) + 8 H,0(1) (a) For the titration, how many moles of MnO, were required if 46.1 mL of 0.154 M KMNO, was needed to reach the end point? Write your answer to the correct number of significant figures. Enter your answer in scientific notation. x 10 mol MnO, (b) How many moles of H,0, were present in the 14.6 g sample of bleach? Write your answer to the correct number of significant figures. mol H;O2
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter15: Equilibria Of Other Reaction Classes
Section: Chapter Questions
Problem 51E: Magnesium metal (a component of alloys used in aircraft and a reducing agent used in the production...
Related questions
Question
Transcribed Image Text:8 https://ezto.mheducation.com/ext/map/index.html?_con=Dcon&external_browser%3D0&launch-
Chapter4-Three Major Classes of Chemical Reactions A
Saved
15
Be sure to answer all parts.
The active agent in many hair bleaches is hydrogen peroxide. The amount of H,O, in 14.6-g of hair
bleach was determined by titration with a standard potassium permanganate solution:
Would
try ano
of this q
2 MnO, (aq) + 5 H,02(aq) + 6 H*(aq) → 5 O2(g) + 2 Mn²*(aq) + 8 H,0(1)
By doing
start from
and lose
(a) For the titration, how many moles of MnO were required if 46.1 mL of 0.154 M KMNO, was
needed to reach the end point? Write your answer to the correct number of significant figures. Enter
your answer in scientific notation.
associated
question.
10
mol MnO
Try an
(b) How many moles of H,0, were present in the 14.6 g sample of bleach? Write your answer to the
correct number of significant figures.
mol H2O2
Mc
Graw
Hill
15 of 30
< Prev
Next >
O Type here to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
