The actual mass of a Eu atom is 150.919846 amu. (Consult Appendix B for any needed reference values. Enter unrounded values for your answers.) (a) Calculate the mass defect in amu/atom and in g/mol for this isotope. amu atom g mol (b) What is the nuclear binding energy in kJ/mol for this isotope? kJ mol
The actual mass of a Eu atom is 150.919846 amu. (Consult Appendix B for any needed reference values. Enter unrounded values for your answers.) (a) Calculate the mass defect in amu/atom and in g/mol for this isotope. amu atom g mol (b) What is the nuclear binding energy in kJ/mol for this isotope? kJ mol
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter19: The Nucleus: A Chemist's View
Section: Chapter Questions
Problem 85CWP
Related questions
Question
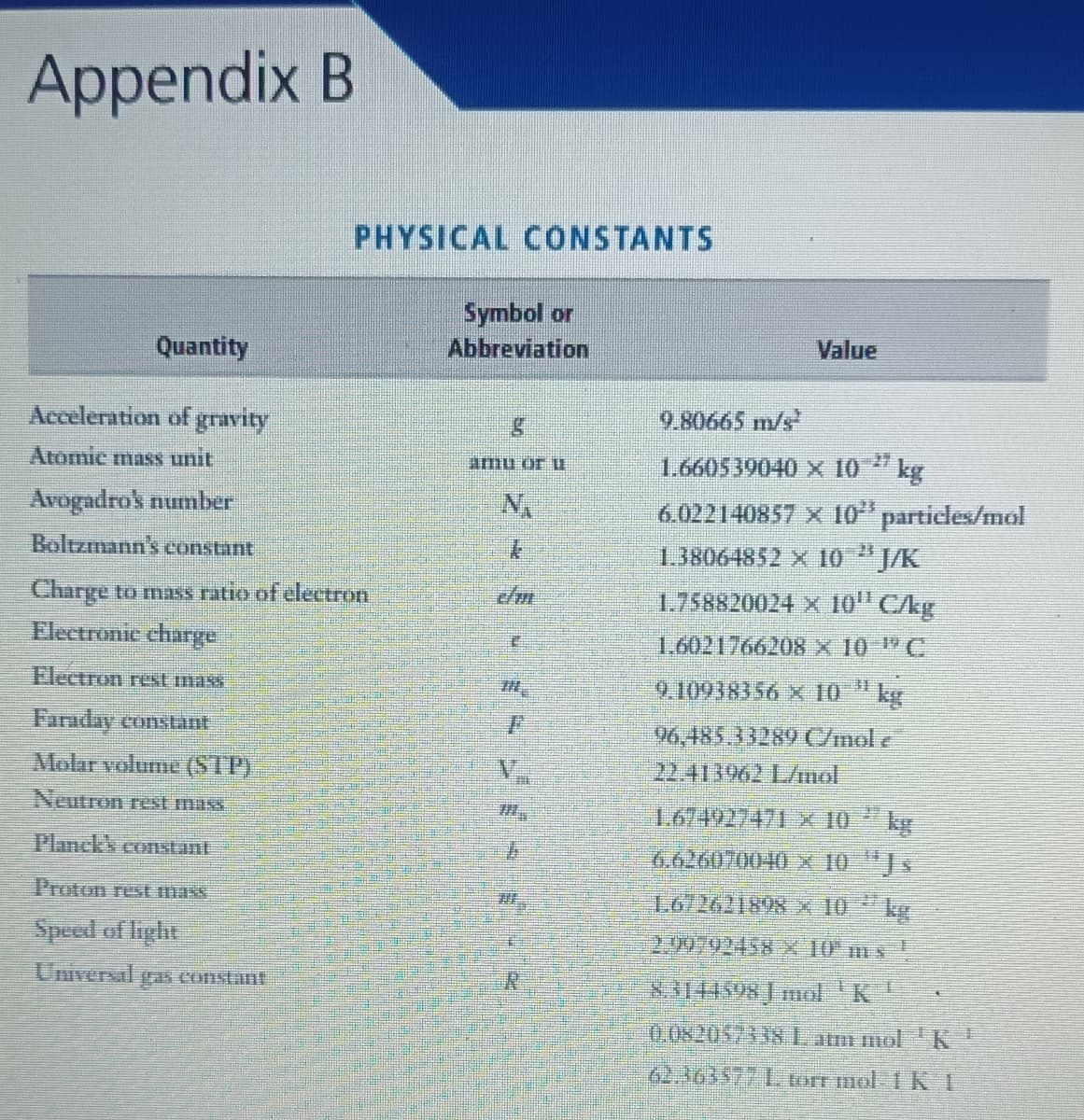
Transcribed Image Text:Appendix B
PHYSICAL CONSTANTS
Symbol or
Abbreviation
Quantity
Value
Acceleration of gravity
9.80665 m/s
Atomie mass unit
1.660539040 x 10
27
Amu or U
kg
6.022140857 X 10" particles/mol
Avogadrok number
Boltzmann's constant
1.38064852 X 10 "J/K
Charge to mas ratio of electron
Electronic charge
1.758820024 X 10" C/kg
1.6021766208 X 10 C
Flectron rest mass
9.10938356 x 10 " kg
Faraday constant
96,485.33289 C/mol e
22.413962 L/imol
Molar volume (STP)
Neutron rest muss
1.674927471- x 10
kg
6.626070040 X 10 "J.
Plhnck's constant
Proton rest mass
1.672621898x 10 kg
Speed of light
2.92792458 x 10 mx
Universal
aS Constant
3144598 mol'K
0,0820575381 atm mol K
62.363577 L torr mol IKI

Transcribed Image Text:The actual mass of a Eu atom is 150.919846 amu (Consult
Appendix B for any needed reference values. Eter unrounded
values for your answers.)
(a) Calculate the mass defect in amu/atom and in g/mol for
this isotope.
amu atom
g/mol
(b) What is the nuclear binding energy in kJ/mol for this
isotope?
kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning