The actual mass of a Sm atom is 146.914893 amu, (Consult Appendix B for any needed reference values. Enter unrounded values for your answers.) (a) Calculate the mass defect in amu/atom and in g/mol for this isotope. amu/atom g/mol (b) What is the nuclear binding energy in kJ/mol for this isotope? kJ/mol
The actual mass of a Sm atom is 146.914893 amu, (Consult Appendix B for any needed reference values. Enter unrounded values for your answers.) (a) Calculate the mass defect in amu/atom and in g/mol for this isotope. amu/atom g/mol (b) What is the nuclear binding energy in kJ/mol for this isotope? kJ/mol
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter2: Matter
Section: Chapter Questions
Problem 28A
Related questions
Question
Take in mind appendix b
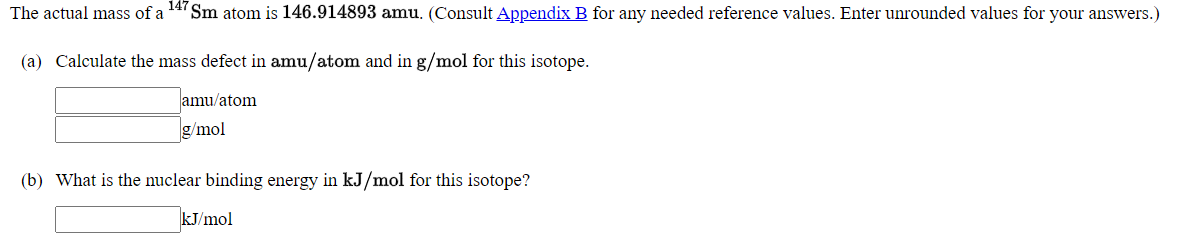
Transcribed Image Text:The actual mass of a
147
Sm atom is 146.914893 amu. (Consult Appendix B for any needed reference values. Enter unrounded values for your answers.)
(a) Calculate the mass defect in amu/atom and in g/mol for this isotope.
amu/atom
g/mol
(b) What is the nuclear binding energy in kJ/mol for this isotope?
kJ/mol
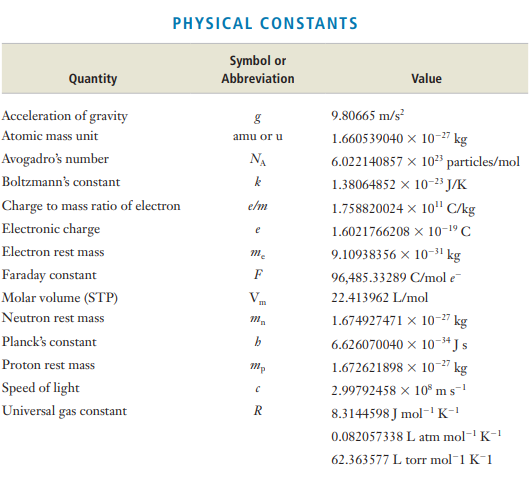
Transcribed Image Text:PHYSICAL CONSTANTS
Symbol or
Quantity
Abbreviation
Value
Acceleration of gravity
9.80665 m/s?
Atomic mass unit
1.660539040 × 10-27 kg
amu or u
Avogadro's number
NA
6.022140857 × 10³ particles/mol
Boltzmann's constant
1.38064852 x 10-23 J/K
Charge to mass ratio of electron
elm
1.758820024 × 10" C/kg
Electronic charge
1.6021766208 x 10-19 C
e
Electron rest mass
9.10938356 x 10-31 kg
Faraday constant
F
96,485.33289 C/mol e-
Molar volume (STP)
V.
22.413962 L/mol
m
Neutron rest mass
1.674927471 x 10-2" kg
Planck's constant
6.626070040 × 10-3*Js
Proton rest mass
1.672621898 x 10" kg
2.99792458 x 10° ms-
8.3144598 J mol-K-!
-27
Speed of light
Universal gas constant
0.082057338 L atm mol-' K-1
62.363577 L torr mol-1 K-1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning



Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co