The addition of hydroiodic acid to a silver nitrate solution precipitates silver iodide according to the reaction: AGNO3 (aq) + HI(aq) → AgI(s) + HNO3 (aq) When 50.0 mL of 5.00x10-2 M AgNO3 is combined with 50.0 mL of 5.00x10-2 M HI in a coffee-cup calorimeter, the temperature changes from 22.40 °C to 22.91 °C. Part A Calculate AHrxn for the reaction as written. Use 1.00 g/mL as the density of the solution and Cs = 4.18 J/(g·°C) as the specific heat capacity of the solution. Express the energy to two significant figures and include the appropriate units. HẢ Units Value ΔΗΚΗ rxn
The addition of hydroiodic acid to a silver nitrate solution precipitates silver iodide according to the reaction: AGNO3 (aq) + HI(aq) → AgI(s) + HNO3 (aq) When 50.0 mL of 5.00x10-2 M AgNO3 is combined with 50.0 mL of 5.00x10-2 M HI in a coffee-cup calorimeter, the temperature changes from 22.40 °C to 22.91 °C. Part A Calculate AHrxn for the reaction as written. Use 1.00 g/mL as the density of the solution and Cs = 4.18 J/(g·°C) as the specific heat capacity of the solution. Express the energy to two significant figures and include the appropriate units. HẢ Units Value ΔΗΚΗ rxn
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 11QAP: When one mol of KOH is neutralized by sulfuric acid, q=56 kJ. (This is called the heat of...
Related questions
Question
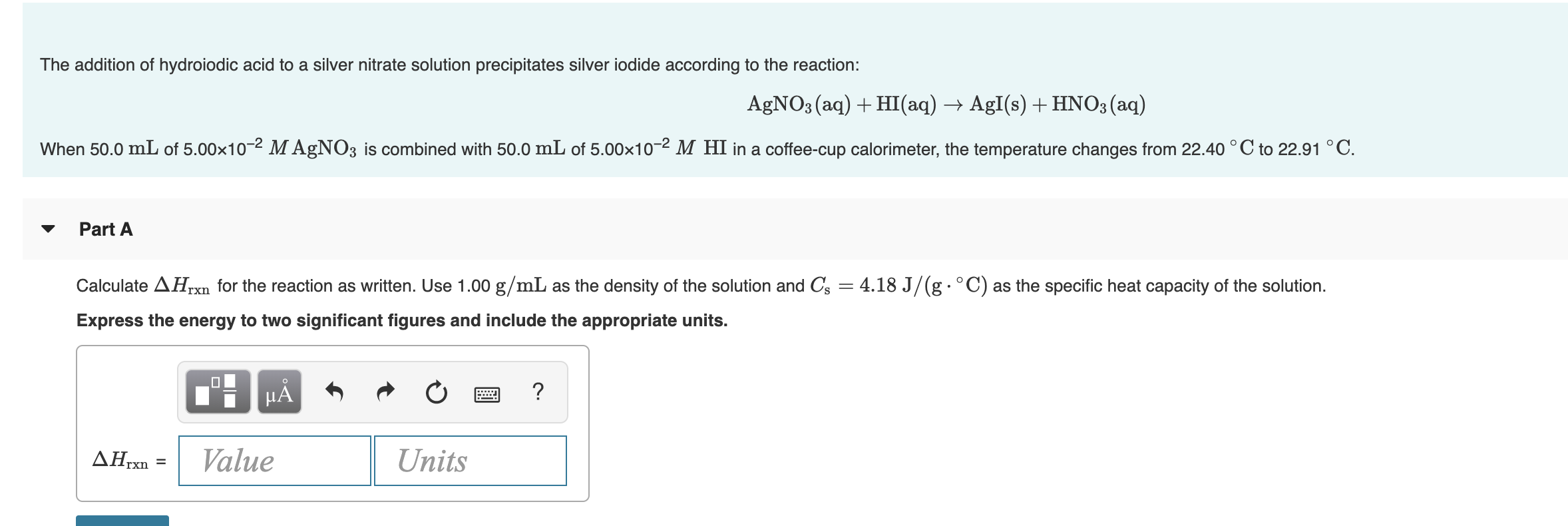
Transcribed Image Text:The addition of hydroiodic acid to a silver nitrate solution precipitates silver iodide according to the reaction:
AGNO3 (aq) + HI(aq) → AgI(s) + HNO3 (aq)
When 50.0 mL of 5.00x10-2 M AgNO3 is combined with 50.0 mL of 5.00x10-2 M HI in a coffee-cup calorimeter, the temperature changes from 22.40 °C to 22.91 °C.
Part A
Calculate AHrxn for the reaction as written. Use 1.00 g/mL as the density of the solution and Cs = 4.18 J/(g·°C) as the specific heat capacity of the solution.
Express the energy to two significant figures and include the appropriate units.
HẢ
Units
Value
ΔΗΚΗ
rxn
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax