The apparatus below contains ammonia, neon, and hydrogen chloride gases at a temperature of 27°C. The valve in the center is opened and the ammonia reacts with the hydrogen chloride. Assume the reaction goes to completion. NH3 (g) + HCl(g) → NH4 Cl(s) a. How many moles of ammonia and HCl are present initially? b. Which reactant is the limiting reactant? c. How many moles of the excess reactant are left over?
The apparatus below contains ammonia, neon, and hydrogen chloride gases at a temperature of 27°C. The valve in the center is opened and the ammonia reacts with the hydrogen chloride. Assume the reaction goes to completion. NH3 (g) + HCl(g) → NH4 Cl(s) a. How many moles of ammonia and HCl are present initially? b. Which reactant is the limiting reactant? c. How many moles of the excess reactant are left over?
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.15P
Related questions
Question
1. The apparatus below contains ammonia, neon, and hydrogen chloride gases at a temperature of 27°C. The valve in the center is opened and the ammonia reacts with the hydrogen chloride. Assume the reaction goes to completion.
NH3 (g) + HCl(g) → NH4 Cl(s)
a. How many moles of ammonia and HCl are present initially?
b. Which reactant is the limiting reactant?
c. How many moles of the excess reactant are left over?
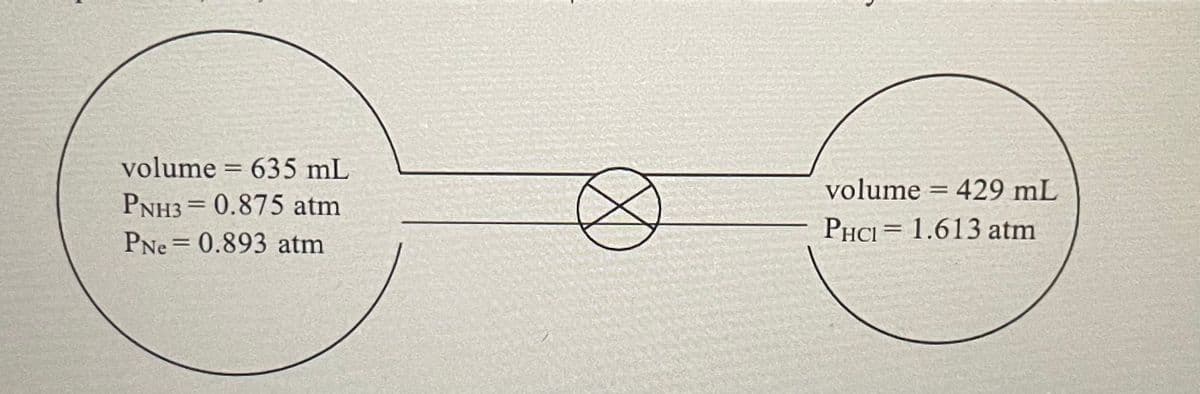
Transcribed Image Text:volume = 635 mL
volume = 429 mL
PNH3= 0.875 atm
PHCI = 1.613 atm
%3D
PNe = 0.893 atm
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning